Certification: SAS Certified Clinical Trials Programmer Using SAS 9
Certification Full Name: SAS Certified Clinical Trials Programmer Using SAS 9
Certification Provider: SAS Institute
Exam Code: A00-281
Exam Name: SAS Clinical Trials Programming Using SAS 9 - Accelerated Version 9 Accelerated Version
Product Screenshots
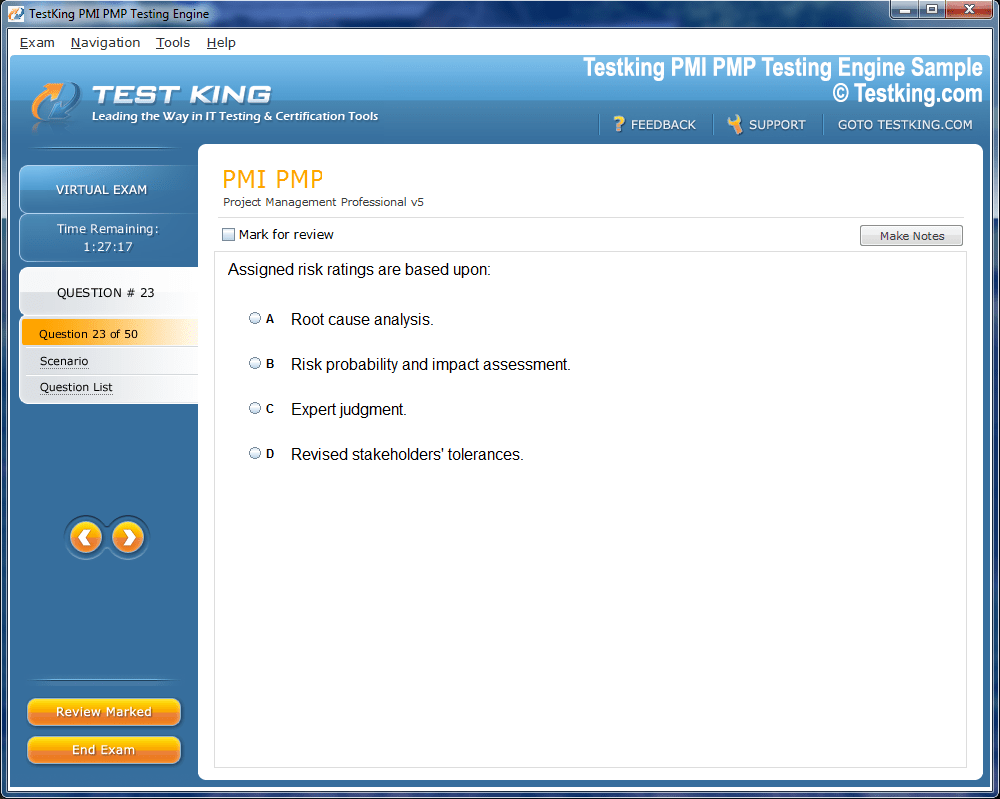
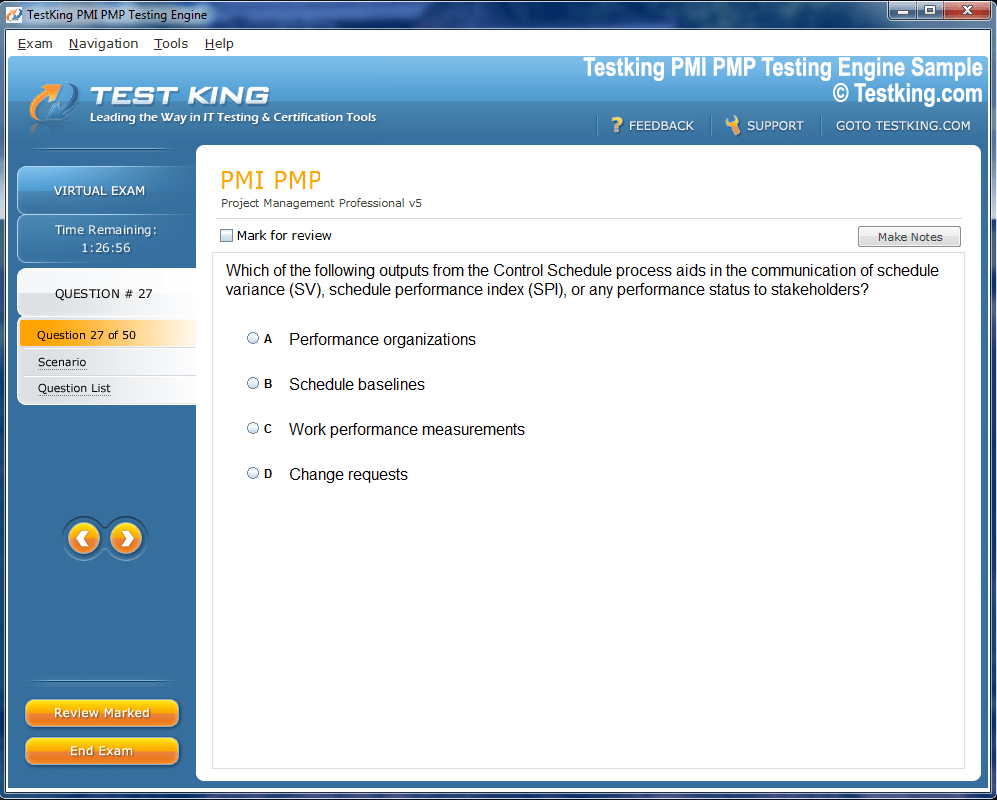
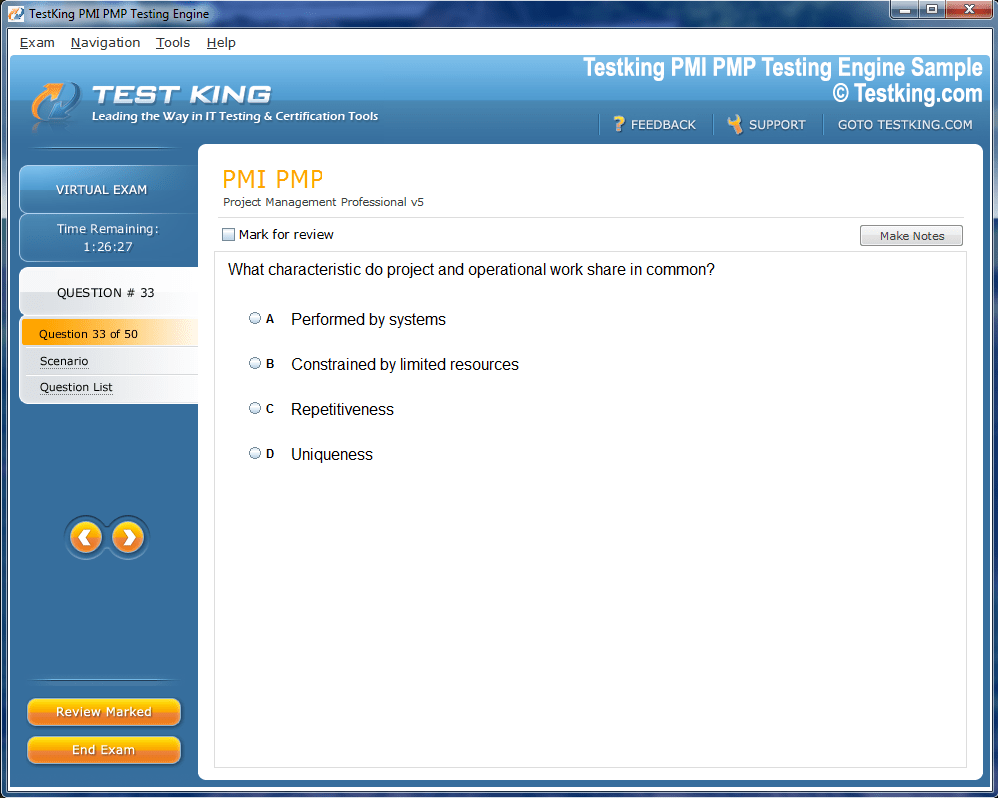
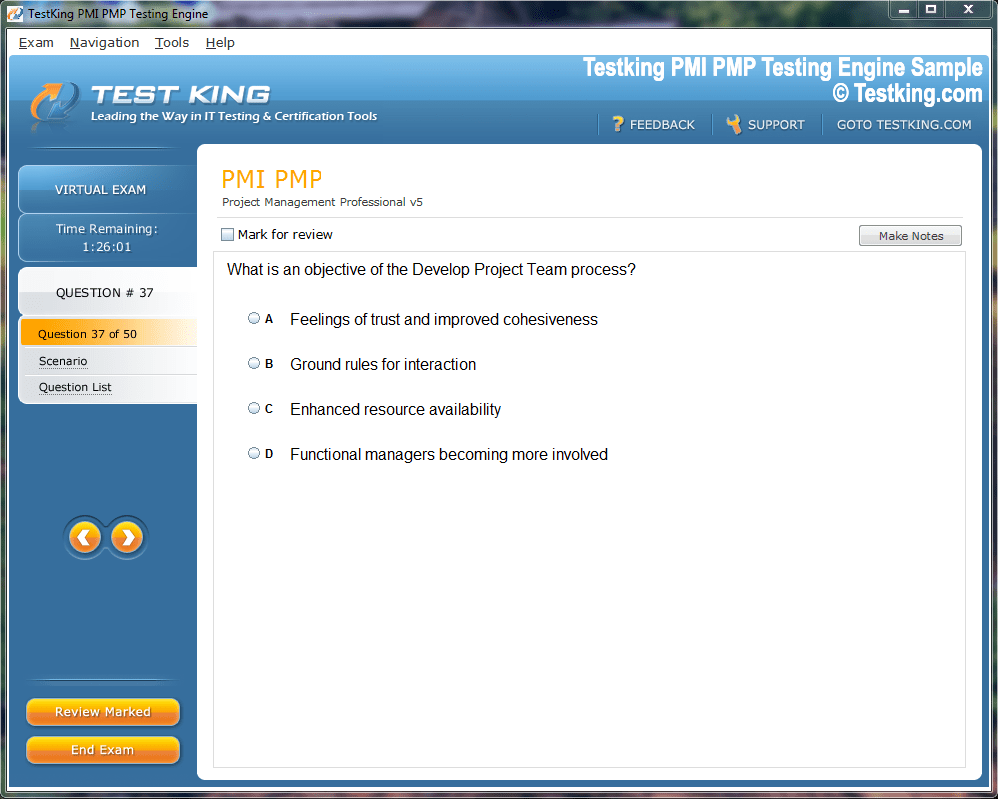
nop-1e =1
Achieving Excellence in Clinical Trials with a SAS Certified Clinical Trials Programmer Using SAS 9 Certification
SAS has long been an indispensable tool for advanced analytics, business intelligence, and data management. Its applications span a multitude of industries, but nowhere is its impact more profound than in the realm of clinical trials. Clinical trials programming entails the meticulous manipulation, analysis, and reporting of complex datasets generated during experimental studies of pharmaceutical products and therapeutic interventions. Professionals who specialize in this domain perform the dual role of data custodian and analyst, ensuring that the vast amounts of information generated in trials are accurate, reliable, and compliant with regulatory standards.
SAS clinical trials programming has evolved to become a highly specialized field. With the integration of global standards such as CDISC, SDTM, and ADaM, programming in clinical trials is no longer just about writing scripts to analyze data. It is about structuring datasets, transforming raw data into usable formats, and generating regulatory-compliant reports that can withstand scrutiny from authorities such as the FDA or EMA. The precision required in this role demands not only proficiency in SAS programming but also a thorough understanding of statistical concepts, clinical trial design, and data governance.
The SAS Clinical Trials Programming Professional certification, recognized within the industry, serves as a benchmark for expertise. It signals to employers and regulatory bodies that the certified individual possesses the technical acumen and practical experience necessary to manage and interpret clinical trial data using SAS 9.4. The exam tests a range of competencies, from basic programming skills to advanced applications, including macro programming, statistical analysis, and data validation techniques. Achieving this certification is often seen as a milestone that elevates a programmer's career trajectory and opens doors to more complex and responsible roles within pharmaceutical organizations.
The Significance of Clinical Trials Programming
Clinical trials are structured investigations designed to evaluate the safety, efficacy, and tolerability of therapeutic interventions. The data generated in these trials are inherently complex, encompassing patient demographics, laboratory measurements, adverse events, treatment outcomes, and longitudinal follow-ups. Transforming these raw datasets into a format suitable for analysis is no trivial task. Each dataset must be scrutinized for inconsistencies, outliers, and missing values. Data cleaning, standardization, and validation are critical processes that ensure the accuracy and integrity of the trial’s conclusions.
SAS programming facilitates this process by offering robust tools for data manipulation, statistical modeling, and reporting. A clinical trials programmer uses SAS to automate repetitive tasks, implement validation checks, and create reusable macros that streamline workflows. The use of structured programming approaches and adherence to industry standards ensures that the data are both reproducible and auditable. In regulated environments, where every analysis could influence regulatory approval and patient safety, the reliability of SAS programs is paramount.
Moreover, clinical trials programming is central to the decision-making process in drug development. Analysts and statisticians rely on the datasets curated by SAS programmers to conclude drug efficacy, identify potential safety concerns, and support submissions to regulatory authorities. Without precise and meticulously programmed datasets, these analyses could be flawed, leading to misinterpretation of results or delays in bringing critical therapies to market.
Core Competencies in Clinical Trials Programming
Mastering SAS clinical trials programming requires a blend of technical, analytical, and domain-specific skills. While foundational programming skills form the baseline, advanced competencies are necessary for managing the intricacies of trial data. These include:
Data Manipulation and Management: Creating, merging, transforming, and validating datasets in accordance with clinical standards. Techniques for handling missing values, identifying outliers, and standardizing variable names are essential.
Statistical Analysis: Applying methods to interpret treatment effects, analyze variance, and summarize outcomes. Understanding statistical concepts ensures that the analyses performed are scientifically sound.
Macro Programming: Developing reusable code modules to automate repetitive tasks, reduce errors, and increase efficiency. Macros are especially useful for generating standardized reports across multiple trials.
Data Reporting: Producing tables, listings, and graphs that clearly communicate trial results to stakeholders and regulatory agencies. Accuracy and clarity in reporting are critical.
Compliance and Standards: Adhering to CDISC standards such as SDTM for raw data organization and ADaM for analysis-ready datasets. Compliance ensures regulatory acceptance and facilitates interoperability across organizations.
Validation and Quality Assurance: Implementing procedures to verify the accuracy of datasets and reports. This includes cross-checking outputs, performing reconciliations, and documenting processes.
These competencies collectively ensure that a clinical trials programmer can navigate the complexities of modern pharmaceutical research while maintaining compliance and delivering reliable, actionable insights.
The SAS Clinical Trials Programming Exam
The certification exam evaluates a professional’s ability to execute the responsibilities outlined above. It contains 60–70 questions, encompassing multiple-choice and short-answer formats, which must be completed within a 110-minute window. A passing score of 68% is required, demonstrating sufficient proficiency across the full spectrum of programming and analytical tasks. The exam fee is set at $180 USD, making it accessible to a broad range of professionals seeking formal recognition of their skills.
The topics covered in the examination are comprehensive and reflect real-world requirements in clinical trials programming. Candidates must be familiar with the entire lifecycle of clinical trials, from protocol design and data collection to regulatory reporting and analysis. A nuanced understanding of data structures, particularly CDISC standards, is critical, as is the ability to manipulate datasets efficiently, apply statistical procedures, and produce high-quality reports. Familiarity with macro programming and automation further distinguishes top performers, as these skills improve productivity and accuracy in routine operations.
Career Implications
Earning a SAS clinical trials programming certification is more than a credential; it represents readiness to contribute meaningfully to the pharmaceutical and biotechnology industries. Professionals in this field are increasingly in demand due to the growth of global clinical research and the emphasis on evidence-based drug development. Career pathways for clinical SAS programmers can lead to senior positions in data management, statistical programming, and clinical data analysis. The work is intellectually stimulating and carries the satisfaction of directly supporting advances in medical science.
Salaries in clinical SAS programming are competitive, reflecting the specialized expertise and responsibility involved. Furthermore, the career offers longevity and mobility, allowing professionals to pivot between organizations, therapeutic areas, or analytical roles. Those who demonstrate proficiency in SAS programming, a keen understanding of trial data, and mastery of regulatory standards are positioned for leadership roles in clinical research, bridging the gap between data management, analysis, and decision-making.
Preparing for a Career in Clinical SAS Programming
Effective preparation for clinical trials programming requires both structured learning and hands-on experience. Aspiring professionals should begin with foundational SAS training, covering data steps, procedures, and basic programming logic. As proficiency grows, emphasis should shift toward clinical trial-specific data structures, regulatory requirements, and advanced SAS techniques, including macros and automated reporting.
Practical experience is invaluable. Working with real-world datasets allows candidates to confront challenges such as missing data, inconsistent coding, and complex variable relationships. This experience reinforces theoretical knowledge and cultivates problem-solving skills essential for success in a regulated environment. Supplementing hands-on practice with study guides, sample exams, and peer interaction enhances comprehension and readiness for certification.
Joining professional forums, study groups, and online communities can provide additional perspectives and insights. Discussions around best practices, troubleshooting, and regulatory expectations help solidify understanding and expose learners to scenarios they may encounter in professional practice. Ultimately, success in clinical trials programming relies on a combination of technical competence, analytical thinking, and familiarity with the regulatory landscape.
SAS clinical trials programming is a multifaceted discipline that sits at the intersection of data analysis, regulatory compliance, and healthcare innovation. It demands precision, analytical acumen, and a deep understanding of both programming and the clinical research domain. Certification as a SAS Clinical Trials Programming Professional validates these skills, offering recognition and career advancement opportunities. As the pharmaceutical industry continues to grow and the demand for accurate, reliable data intensifies, expertise in SAS clinical trials programming will remain a vital and highly valued capability.
Eligibility and Prerequisites for SAS Clinical Trials Programming Professional
SAS clinical trials programming is a specialized discipline that necessitates not only technical expertise but also domain-specific experience in managing clinical datasets. The SAS Clinical Trials Programming Professional certification validates this combination of skills and experience. Achieving this certification requires candidates to meet certain prerequisites, which ensure that they possess the foundational knowledge and practical exposure necessary to succeed in a regulated and complex environment.
Prerequisite Certifications
To be eligible for the SAS Clinical Trials Programming Professional credential, candidates must already hold specific certifications. The Base Programming Specialist and the Advanced Programming Professional certifications are recognized as essential prerequisites. These credentials establish a candidate’s ability to handle the foundational aspects of SAS programming, including data step processing, macro creation, and advanced procedures. By demonstrating competence at this level, candidates confirm that they can approach the more intricate requirements of clinical trials programming with confidence.
The Base Programming Specialist certification equips professionals with knowledge of essential SAS programming constructs. Topics such as data step execution, variable manipulation, dataset merging, and basic procedures like PROC SORT and PROC PRINT form the cornerstone of SAS competency. Building upon this foundation, the Advanced Programming Professional certification introduces more sophisticated capabilities, including advanced data transformations, macro development, and SQL integration within SAS. This layered approach ensures that candidates have the requisite skills before transitioning to the highly specialized domain of clinical trials data management and reporting.
Recommended Training and Experience
In addition to holding prerequisite certifications, candidates are encouraged to pursue structured training courses or possess equivalent practical experience. Recommended SAS training programs provide in-depth coverage of topics critical to clinical trials programming, including the organization of clinical datasets, adherence to CDISC standards, and the generation of regulatory-compliant reports. While formal training is advantageous, equivalent professional experience can substitute for classroom instruction, provided it exposes the candidate to real-world clinical trial datasets and programming scenarios.
Hands-on experience is crucial in developing the analytical rigor and programming discipline needed for clinical trials. Candidates should have a minimum of six months working directly with SAS in a clinical research context. This period allows for immersion in the nuances of clinical datasets, the exploration of regulatory documentation requirements, and the development of strategies for efficient data handling. During this time, candidates encounter the typical challenges of clinical programming, such as handling missing or inconsistent data, transforming raw datasets into analysis-ready formats, and producing tables and graphs that meet both statistical and regulatory standards.
Practical Experience in Clinical Trials Programming
Practical experience serves as the bridge between theoretical knowledge and professional proficiency. Clinical trials datasets often include demographic information, laboratory results, adverse event logs, and efficacy measures collected at multiple time points. Working with such datasets allows candidates to refine their skills in data cleaning, restructuring, and summarization.
Experience in generating regulatory-compliant reports is equally critical. Reporting requirements are stringent, and inaccuracies or inconsistencies can compromise the integrity of a clinical trial submission. Programmers must develop familiarity with the formats and conventions of tables, listings, and figures (TLFs) commonly required in regulatory submissions. Developing proficiency in these outputs through repeated practice ensures that candidates can meet industry standards with precision and efficiency.
Understanding Industry Standards
Clinical trials programming is governed by widely accepted industry standards that facilitate consistency, interoperability, and regulatory compliance. The Clinical Data Interchange Standards Consortium (CDISC) provides comprehensive guidelines for data structure and submission formats. Within CDISC, the Study Data Tabulation Model (SDTM) organizes raw clinical data into standardized domains, making datasets easier to interpret and analyze. The Analysis Data Model (ADaM) further refines datasets for statistical analysis, ensuring that derived variables, analysis datasets, and metadata align with regulatory expectations.
Proficiency in these standards is a fundamental requirement for SAS clinical trials programmers. Candidates must understand how to map raw trial data to SDTM domains, create ADaM datasets for statistical analysis, and maintain traceability between raw and derived variables. Knowledge of these frameworks ensures that datasets are structured logically, can be audited, and are readily usable by statisticians and regulatory reviewers.
Core Skills for Eligibility
In addition to formal prerequisites, successful candidates typically demonstrate a combination of technical skills and analytical capabilities. Core skills include:
Data Step Mastery: Understanding the nuances of SAS data steps, including conditional processing, variable manipulation, and iterative data transformations.
Procedures Proficiency: Efficient use of procedures such as PROC SORT, PROC TRANSPOSE, PROC REPORT, and PROC MEANS to prepare and analyze datasets.
Macro Development: Creating reusable code structures that streamline data processing and report generation. Macros improve consistency and reduce the risk of human error.
Data Validation Techniques: Implementing systematic checks to identify discrepancies, ensure consistency, and confirm data integrity. Validation also includes comparing datasets and reconciling discrepancies between source data and analysis-ready datasets.
Statistical Awareness: Applying basic statistical procedures relevant to clinical trials, including frequency distributions, summary statistics, and exploratory analyses. Understanding statistical concepts allows programmers to generate datasets and reports that support reliable conclusions.
By mastering these skills, candidates are better prepared to handle the responsibilities of a clinical trials programmer and to succeed in the certification examination.
Benefits of Meeting Eligibility Requirements
Meeting eligibility requirements provides several advantages. First, it ensures that candidates possess a solid foundation in SAS programming, reducing the likelihood of encountering knowledge gaps during preparation. Second, prior training and experience familiarize candidates with the workflows, standards, and conventions used in clinical trials, making exam questions more intuitive. Third, professional exposure to real-world datasets and reporting practices enhances confidence, allowing candidates to approach the exam with practical insights rather than solely theoretical understanding.
Moreover, eligibility requirements help maintain a high standard within the field. Employers, regulators, and peers recognize that individuals holding the SAS Clinical Trials Programming Professional certification have undergone rigorous preparation, possess relevant experience, and adhere to recognized industry standards. This recognition adds credibility to the professional’s profile and can facilitate career advancement, professional mobility, and participation in complex clinical research projects.
Preparing for Certification
Candidates should approach certification with a structured plan that combines theory, practice, and review. Starting with a thorough examination of the syllabus ensures clarity regarding the knowledge domains that will be tested. Focusing on areas such as data structures, reporting, statistical procedures, and macro programming allows for targeted preparation.
Practical application is indispensable. Candidates should engage with sample datasets, simulate typical clinical trial scenarios, and practice creating SDTM-compliant domains and ADaM analysis datasets. Generating tables, listings, and graphs for regulatory submission scenarios reinforces familiarity with real-world expectations. Additionally, practicing data validation procedures, performing reconciliations, and automating repetitive tasks using macros cultivates both efficiency and accuracy.
Study aids such as guides, tutorials, and sample exams provide a structured path to review and self-assessment. While preparation materials vary in complexity, the most effective resources combine conceptual explanations with hands-on exercises. Connecting with peers, participating in forums, or joining study groups offers further opportunities to discuss challenges, clarify doubts, and share practical insights.
Career Implications of Meeting Eligibility
Meeting the eligibility requirements is not solely about gaining access to the certification exam. It also sets the stage for a sustainable and rewarding career. Professionals who satisfy prerequisites are well-positioned to contribute meaningfully to clinical trial projects, manage datasets efficiently, and produce reports that withstand regulatory scrutiny.
Eligibility requirements also enhance career mobility. Certified professionals can explore opportunities across different therapeutic areas, trial phases, and geographic regions. The structured preparation and hands-on experience gained during the eligibility phase equip programmers with skills that are transferable to other analytical roles, including biostatistics, data management, and pharmacovigilance.
Eligibility and prerequisites form the foundation for success in SAS clinical trials programming. By holding the necessary certifications, completing recommended training, and gaining practical experience, candidates establish a robust skill set that allows them to navigate the complexities of clinical datasets, regulatory requirements, and reporting standards. Understanding industry conventions, mastering SAS programming techniques, and applying practical experience collectively enhance readiness for the SAS Clinical Trials Programming Professional certification.
Meeting these criteria ensures that candidates are not only prepared for the examination but also capable of contributing to the high-stakes environment of clinical research. As the demand for skilled SAS programmers continues to grow, meeting eligibility requirements becomes an investment in professional growth, technical expertise, and career longevity. Professionals who adhere to these standards are recognized as competent, reliable, and integral contributors to the success of clinical trials and the broader pharmaceutical research ecosystem.
Clinical Trials Data Structures and Advanced SAS Programming Techniques
Clinical trials programming is a highly specialized discipline that relies on both technical expertise and domain-specific knowledge. At the heart of this specialization is the effective organization, transformation, and analysis of clinical trial data. Understanding the data structures employed in clinical trials, coupled with mastery of advanced SAS programming techniques, is fundamental for ensuring data integrity, regulatory compliance, and accurate reporting.
The Importance of Data Structures in Clinical Trials
Clinical trials generate voluminous and heterogeneous datasets encompassing patient demographics, laboratory measurements, adverse events, treatment responses, and longitudinal follow-up information. Properly organizing this data is essential to maintain clarity, ensure reproducibility, and facilitate statistical analysis. Clinical trial data structures provide a standardized framework to manage these complexities and enable interoperability across teams, studies, and organizations.
The Clinical Data Interchange Standards Consortium (CDISC) has established widely adopted standards that dictate how clinical trial data should be structured and reported. Among these, the Study Data Tabulation Model (SDTM) and the Analysis Data Model (ADaM) are central. SDTM organizes raw trial data into domains that reflect study parameters, whereas ADaM prepares datasets specifically for statistical analysis. Adhering to these standards ensures that datasets are intelligible to both statisticians and regulatory authorities and can withstand rigorous audits and reviews.
Study Data Tabulation Model (SDTM)
SDTM provides a uniform structure for raw clinical data, facilitating interpretation, traceability, and submission to regulatory agencies. Each SDTM dataset corresponds to a specific domain, such as demographics (DM), adverse events (AE), or laboratory results (LB). Within these domains, variables are standardized, units are harmonized, and metadata are clearly documented.
For SAS programmers, understanding SDTM involves not only creating datasets according to the specified domains but also ensuring consistency, completeness, and compliance with controlled terminology. Mapping raw data to SDTM domains requires attention to detail, including reconciling discrepancies, managing missing values, and applying derivations when necessary. Efficient SAS code can automate many of these transformations, improving accuracy and reducing the risk of manual error.
Analysis Data Model (ADaM)
While SDTM focuses on raw data organization, ADaM structures datasets for statistical analysis and regulatory reporting. ADaM datasets incorporate derived variables, analysis flags, and metadata that enable reproducible statistical results. Variables in ADaM are defined to support specific endpoints, treatment comparisons, and statistical models.
Creating ADaM datasets requires the ability to manipulate data, perform calculations, and maintain traceability to SDTM datasets. SAS programmers employ advanced procedures, macros, and conditional logic to generate analysis-ready datasets efficiently. Mastery of ADaM is particularly critical for ensuring that analyses are auditable, reproducible, and compliant with regulatory standards.
Advanced SAS Programming Techniques
In addition to understanding clinical trial data structures, advanced SAS programming skills are essential for efficiency, automation, and accuracy. These techniques allow programmers to handle complex datasets, generate standardized reports, and implement quality checks.
Macro Programming
Macro programming is a cornerstone of advanced SAS programming. Macros enable the creation of reusable code modules that automate repetitive tasks, such as data cleaning, variable derivation, and report generation. By parameterizing macros, programmers can apply identical procedures across multiple datasets or studies, ensuring consistency while reducing the likelihood of error.
Effective macro programming also involves designing modular code that is easy to maintain and debug. Well-structured macros enhance productivity and allow teams to standardize procedures across clinical trial projects, facilitating collaboration and regulatory compliance.
Data Transformation Techniques
Transforming raw data into analysis-ready formats often requires complex manipulations. Techniques include transposing datasets, merging multiple sources, creating derived variables, and performing conditional computations. SAS procedures such as PROC TRANSPOSE, PROC SORT, and PROC SQL are commonly used in these transformations.
Understanding the nuances of data transformation is critical. For example, handling repeated measures, time-to-event variables, or hierarchical data structures requires careful programming to maintain the integrity of relationships between variables. Advanced techniques also encompass array processing, iterative loops, and conditional logic to streamline the processing of large and complex datasets.
Statistical Procedures
Clinical trials programming is intrinsically linked to statistical analysis. SAS programmers apply a range of procedures to summarize, explore, and validate trial data. Procedures such as PROC MEANS, PROC FREQ, and PROC UNIVARIATE allow for descriptive statistics, frequency analyses, and distributional assessments. More sophisticated procedures, including PROC GLM or PROC MIXED, support analyses for longitudinal studies, covariate adjustments, and multivariate models.
A critical aspect of these procedures is ensuring that the outputs are interpretable, accurate, and ready for inclusion in regulatory submissions. Programmers must understand both the statistical concepts underpinning the procedures and the practical implementation details in SAS. This dual understanding ensures that results reflect the intended analyses and are consistent with trial objectives.
Quality Assurance and Validation
Advanced SAS programming is incomplete without rigorous quality assurance and validation processes. Clinical trial datasets and reports must undergo systematic verification to confirm accuracy, consistency, and regulatory compliance.
Validation techniques include cross-checking datasets against source data, comparing outputs with expected results, and reviewing derivation algorithms. Programmers often employ automated validation scripts, which can detect discrepancies, missing values, or incorrect derivations. These practices are essential for minimizing errors and ensuring the reliability of clinical trial conclusions.
Reporting and Documentation
Generating reports is a central responsibility of clinical trials programmers. Tables, listings, and graphs (TLFs) provide clear communication of trial results to statisticians, clinical teams, and regulatory authorities. Advanced SAS techniques, such as PROC REPORT, PROC TABULATE, and ODS GRAPHICS, allow for the creation of publication-ready outputs that adhere to regulatory standards.
Documentation is equally important. Each dataset, macro, and output must be accompanied by metadata and annotations that explain the methodology, assumptions, and derivations used. This documentation ensures transparency, reproducibility, and audit readiness, all of which are crucial in regulated environments.
Integrating Data Standards with Advanced Programming
The synergy between clinical trial data standards and advanced SAS programming techniques is the hallmark of proficient clinical trials programming. SDTM and ADaM provide a blueprint for structuring data, while macros, procedures, and transformation techniques enable efficient execution. By integrating these components, programmers create a workflow that is both scalable and compliant.
For example, a programmer may use a macro to standardize SDTM domains across multiple studies, ensuring that variable names, units, and controlled terminologies are consistent. Once standardized, data can be transformed into ADaM datasets using advanced procedures and conditional logic. Subsequent statistical analyses and report generation then leverage these standardized datasets, creating a seamless pipeline from raw data to regulatory submission.
Practical Applications in the Workplace
In practice, SAS clinical trials programmers encounter datasets of varying complexity. Early-phase studies may involve smaller datasets with straightforward structures, whereas late-phase or multi-center trials can generate massive, multi-dimensional datasets requiring sophisticated transformations and analyses.
Programmers must navigate these complexities while maintaining adherence to regulatory standards. They collaborate closely with statisticians, data managers, and clinical teams to ensure that datasets are accurate, analysis-ready, and properly documented. The ability to adapt advanced SAS techniques to diverse trial scenarios is a distinguishing feature of experienced clinical trials programmers.
Continuous Learning and Skill Enhancement
Clinical trials programming is a dynamic field, with evolving standards, emerging statistical methodologies, and continual software updates. Professionals must engage in continuous learning to remain proficient. This includes staying current with changes in CDISC standards, exploring new SAS procedures and features, and expanding expertise in statistical methodologies relevant to clinical research.
Participating in professional forums, attending workshops, and collaborating with peers are effective strategies for skill enhancement. Exposure to real-world challenges and diverse datasets deepens understanding, refines problem-solving abilities, and cultivates adaptability, which is essential in a field where precision and accuracy are paramount.
Mastery of clinical trials data structures and advanced SAS programming techniques is central to the role of a clinical trials programmer. Understanding SDTM and ADaM standards provides a framework for organizing and analyzing data, while macro programming, data transformation, and statistical procedures enable efficient execution of complex tasks.
Validation, quality assurance, and documentation ensure the integrity and reliability of datasets and outputs, creating a foundation for reproducible, auditable results. By integrating these competencies, SAS programmers support clinical trials from data collection to regulatory submission, contributing to the development of safe and effective therapeutic interventions.
Advanced programming skills, combined with adherence to standardized data structures, allow professionals to navigate the intricacies of clinical trial datasets with confidence and precision. As the pharmaceutical and biotechnology sectors continue to expand, proficiency in these areas remains critical, reinforcing the significance of SAS clinical trials programming as a specialized and highly valued discipline.
Statistical Analysis, Regulatory Submissions, and Reporting in Clinical Trials Programming
Clinical trials programming involves more than simply managing datasets; it requires the ability to perform sophisticated statistical analyses, generate regulatory-compliant reports, and ensure the integrity of all outputs. SAS programmers serve as a critical link between raw clinical data and the interpretation of results, providing structured, validated information that informs decision-making in pharmaceutical development. Mastery of statistical procedures, an understanding of regulatory requirements, and proficiency in reporting techniques are fundamental competencies for professionals in this domain.
Role of Statistical Analysis in Clinical Trials
Statistical analysis is central to evaluating the efficacy and safety of therapeutic interventions. SAS programmers collaborate with statisticians to prepare datasets that can support hypothesis testing, safety assessments, and outcome interpretations. The analyses range from simple descriptive statistics to complex models that account for repeated measures, covariates, and longitudinal data.
Key statistical objectives in clinical trials programming include summarizing patient demographics, identifying treatment effects, detecting adverse event trends, and ensuring that endpoints are evaluated according to the study protocol. Accurate statistical outputs depend on well-structured datasets and reliable programming practices. SAS provides an array of procedures that facilitate these analyses, including PROC MEANS for descriptive summaries, PROC FREQ for frequency distributions, PROC UNIVARIATE for assessing distributions, and PROC GLM or PROC MIXED for advanced modeling.
A critical aspect of statistical analysis is ensuring reproducibility and auditability. Each analysis must be traceable to source data, derivations must be documented, and any assumptions or transformations must be clearly defined. SAS programmers play an essential role in maintaining this chain of transparency, preparing datasets and analysis outputs that are both methodologically sound and compliant with regulatory expectations.
Integration with Regulatory Submissions
The pharmaceutical industry operates within a stringent regulatory framework designed to safeguard patient safety and ensure the validity of clinical findings. Regulatory submissions, such as New Drug Applications (NDAs) or Biologics License Applications (BLAs), require precise, well-documented datasets and analytical outputs. SAS programmers are responsible for preparing the underlying data, analyses, and reports that support these submissions.
Adherence to CDISC standards is critical for regulatory acceptance. SDTM datasets provide standardized raw data, while ADaM datasets contain analysis-ready data for statistical evaluation. Regulatory agencies expect traceability between these datasets, which allows reviewers to follow the derivation of analysis results from source observations. SAS programmers must ensure that all datasets, programs, and outputs are thoroughly validated, properly annotated, and compliant with both internal and regulatory quality standards.
In addition to standard datasets, programmers often generate supplemental documentation, including metadata, specifications, and annotated datasets. These documents provide context, clarify derivations, and demonstrate adherence to submission guidelines. The combination of structured datasets, reproducible code, and comprehensive documentation forms the backbone of regulatory submissions, emphasizing the programmer’s role as both analyst and custodian of data integrity.
Data Cleaning and Validation
Before statistical analyses and reporting, datasets must undergo rigorous cleaning and validation. Clinical trial data are inherently complex, often containing missing values, inconsistencies, outliers, and errors introduced during data collection. SAS programmers implement systematic checks to identify and correct these issues, ensuring that datasets are accurate and reliable.
Common validation techniques include cross-referencing data with source documents, comparing derived variables with expected values, and applying logical consistency checks across related datasets. Automated validation scripts, often developed using macros, enhance efficiency and reduce the potential for human error. Effective validation ensures that subsequent statistical analyses and reports reflect true clinical findings rather than artifacts of data discrepancies.
Reporting Clinical Trial Results
Reporting is a fundamental responsibility of SAS clinical trials programmers. The creation of tables, listings, and graphs (TLGs) transforms raw and analyzed data into digestible, interpretable formats for clinicians, statisticians, and regulatory reviewers. Reports must be accurate, consistent, and clearly convey the outcomes of the trial.
SAS provides a range of tools to generate high-quality reports. PROC REPORT and PROC TABULATE facilitate structured tables with summary statistics and categorical breakdowns. PROC SGPLOT and ODS GRAPHICS enable graphical representation of trends, distributions, and comparative analyses. The outputs are designed to meet regulatory standards, with attention to formatting, labeling, and clarity.
In addition to regulatory reporting, these outputs support internal decision-making. Clinical teams rely on TLGs to monitor trial progress, identify emerging safety signals, and adjust study protocols when necessary. Accurate reporting thus serves a dual purpose: regulatory compliance and operational insight, highlighting the strategic importance of SAS programmers in clinical trials.
Advanced Reporting Techniques
Advanced reporting techniques extend beyond basic tables and graphs. SAS macros are often employed to standardize reporting processes across multiple studies or sites, ensuring consistency and reducing manual effort. Parameterized macros can produce complex outputs dynamically, allowing for rapid adaptation to new datasets or evolving trial requirements.
Programmers also integrate statistical results directly into reports, automating the creation of tables that summarize key endpoints, subgroup analyses, and adverse events. These techniques require proficiency in both SAS programming and clinical trial methodology, as outputs must be scientifically accurate, methodologically justified, and visually clear.
Ensuring Compliance and Quality Assurance
Regulatory compliance and quality assurance are non-negotiable aspects of clinical trials programming. Every dataset, program, and report must withstand scrutiny from auditors and regulatory authorities. SAS programmers implement robust quality assurance processes, including peer reviews, code inspections, and automated validation checks.
These practices ensure that datasets are complete, analyses are accurate, and reports are consistent with the study protocol. Quality assurance also extends to metadata management, where programmers document variable definitions, derivation algorithms, and analytical methods. Comprehensive documentation not only facilitates internal review but also supports regulatory audits and inspections, reinforcing the credibility and reliability of the clinical trial data.
Handling Complex Datasets
Complex datasets are a common feature of modern clinical trials. Multi-center trials, longitudinal studies, and trials with multiple treatment arms generate multi-dimensional data requiring sophisticated programming approaches. SAS programmers must adeptly handle hierarchical datasets, repeated measures, and time-to-event variables while maintaining data integrity and compliance with standards.
Advanced SAS techniques, including array processing, iterative loops, and conditional logic, enable efficient manipulation of these complex datasets. Proper documentation, automated validation, and adherence to data standards are essential to ensure that the derived datasets are accurate, auditable, and ready for statistical analysis.
Collaboration with Clinical and Statistical Teams
Clinical trials programming is inherently collaborative. Programmers work closely with statisticians, clinical researchers, and data managers to ensure that datasets meet study objectives, regulatory requirements, and analytical needs. Collaboration involves understanding the study protocol, translating statistical analysis plans into datasets and programs, and providing feedback on data quality and consistency.
Effective communication and collaboration enhance the accuracy and efficiency of data processing and reporting. SAS programmers must not only execute technical tasks but also interpret clinical and statistical requirements, ensuring that datasets, analyses, and reports align with the intended objectives of the trial.
Continuous Learning and Adaptation
The field of clinical trials programming is dynamic, with evolving statistical methodologies, regulatory standards, and software capabilities. Professionals must engage in continuous learning to remain proficient. This includes staying current with updates to CDISC standards, exploring new SAS procedures and features, and expanding expertise in statistical techniques relevant to clinical research.
Professional development also involves refining programming practices, adopting automation strategies, and exploring innovative reporting techniques. Exposure to diverse trial designs, therapeutic areas, and data complexities enhances adaptability, problem-solving skills, and technical proficiency, which are critical for long-term success in clinical trials programming.
Practical Applications in Regulatory Submissions
In practice, SAS programmers support regulatory submissions through meticulous preparation of SDTM and ADaM datasets, comprehensive validation, and precise reporting. Programs are developed to automate routine tasks, generate derivations, and standardize outputs across multiple datasets. These practices ensure that submissions are reproducible, auditable, and aligned with regulatory expectations.
Programmers also assist in creating supplemental documentation, including annotated datasets, metadata specifications, and derivation notes. This documentation clarifies the methodology, maintains traceability, and demonstrates adherence to standards, facilitating regulatory review and expediting the approval process.
Statistical analysis, regulatory submissions, and reporting form the core of clinical trials programming. Proficiency in these areas requires not only technical expertise in SAS but also an understanding of clinical trial methodology, regulatory expectations, and quality assurance practices.
SAS programmers play a pivotal role in transforming raw clinical data into validated, analysis-ready datasets and interpretable reports. Their work ensures that clinical trials yield reliable, reproducible, and regulatory-compliant results, ultimately supporting the development of safe and effective therapies.
By mastering statistical procedures, reporting techniques, and regulatory requirements, programmers contribute to the integrity, transparency, and success of clinical research. Continuous learning, practical experience, and adherence to data standards ensure that professionals remain at the forefront of this critical and evolving field, reinforcing the essential role of SAS clinical trials programming within the pharmaceutical and biotechnology industries.
Macro Programming, Data Transformation, Validation, and Optimization in Clinical Trials Programming
Clinical trials programming demands more than a foundational knowledge of SAS; it requires advanced technical skills to efficiently manipulate complex datasets, automate repetitive tasks, validate results, and optimize workflows. Macro programming, data transformation, and rigorous validation are central to achieving accuracy, reproducibility, and efficiency in this highly regulated environment.
The Role of Macro Programming in Clinical Trials
Macro programming is a defining feature of advanced SAS programming. Macros allow programmers to encapsulate repetitive code, create parameterized workflows, and standardize processes across multiple datasets or studies. In clinical trials, where similar tasks are performed across multiple phases, sites, or treatment arms, macros save significant time while ensuring consistency.
For example, generating tables, listings, and figures (TLFs) for multiple clinical trial arms can be automated using macros. Instead of manually coding each report, a parameterized macro can accept inputs such as study arm, treatment group, or visit schedule and produce standardized outputs. This approach reduces errors, enhances reproducibility, and allows programmers to focus on more complex analytical tasks.
Macro programming also supports quality assurance. By embedding validation steps and error checks within macros, programmers can ensure that transformations and derivations are applied correctly. Advanced macro techniques, including conditional processing, iterative loops, and dynamic code generation, are essential for managing the intricate and voluminous datasets typical in clinical trials.
Data Transformation Techniques
Transforming raw clinical trial data into structured, analysis-ready formats is a central responsibility of SAS programmers. Data transformation involves tasks such as merging multiple datasets, creating derived variables, aggregating measurements, and reshaping data structures to meet SDTM or ADaM standards.
SAS provides a suite of tools for these transformations. PROC SORT and PROC SQL facilitate dataset merging and ordering, while PROC TRANSPOSE allows reshaping of longitudinal or repeated measures data. Data step programming is used to derive new variables, recode categorical values, and apply conditional transformations. These techniques enable programmers to prepare datasets that are consistent, standardized, and ready for statistical analysis or reporting.
Efficiency in data transformation is critical. Clinical trials often generate large datasets with thousands of variables and hundreds of thousands of records. Advanced techniques such as array processing, hash objects, and iterative loops allow programmers to process data efficiently, minimizing runtime while maintaining accuracy. Properly designed data transformations also support traceability, ensuring that derivations are documented and reproducible.
Validation and Quality Assurance
Validation is a cornerstone of clinical trials programming. Regulatory authorities and internal quality teams require that datasets, derivations, and reports be accurate, consistent, and compliant with standards. SAS programmers implement systematic validation procedures to ensure the integrity of data and outputs.
Validation techniques include:
Cross-checking datasets: Comparing source and derived datasets to identify discrepancies or missing values.
Reconciliation of derived variables: Ensuring that calculations, flags, and indicators align with specifications and study protocols.
Automated validation scripts: Embedding checks within macros or SAS procedures to flag inconsistencies, outliers, or unexpected values.
Peer review and code inspection: Reviewing programs to identify logical errors, improve efficiency, and verify adherence to coding standards.
Effective validation protects against errors that could compromise statistical analyses or regulatory submissions. By combining automated checks with manual review, programmers create datasets and reports that are both reliable and auditable.
Optimizing Clinical Trials Workflows
Optimization in clinical trials programming refers to improving the efficiency, scalability, and maintainability of SAS workflows. This is essential when handling large-scale studies, multi-center trials, or longitudinal data with complex structures.
Workflow optimization strategies include:
Macro utilization: Automating repetitive tasks reduces manual coding, minimizes errors, and accelerates reporting.
Code modularization: Breaking code into reusable modules or functions improves maintainability and readability.
Efficient data processing: Utilizing PROC SQL, arrays, and hash objects to optimize runtime for large datasets.
Standardized templates: Creating templates for datasets, reports, and derivations ensures consistency across studies.
Documentation and annotation: Maintaining comprehensive metadata and program documentation supports reproducibility and audit readiness.
Optimized workflows not only save time but also enhance compliance and quality. They enable SAS programmers to focus on complex analyses, innovative solutions, and regulatory reporting, rather than repetitive coding tasks.
Handling Large and Complex Datasets
Modern clinical trials often involve vast and multidimensional datasets. These may include longitudinal patient measurements, genomic data, imaging results, or data from multiple study centers. Efficiently processing and managing these datasets requires a combination of advanced programming techniques and strategic workflow planning.
Array processing and hash objects are particularly useful for handling large datasets. Arrays allow programmers to perform operations across multiple variables simultaneously, while hash objects facilitate rapid lookups and joins. Conditional processing and iterative loops enable complex transformations and derivations without sacrificing efficiency.
Understanding the structure and relationships within the data is equally important. Programmers must account for repeated measures, hierarchical data, and time-to-event variables while maintaining compliance with CDISC standards and traceability to source data. Proper handling of complex datasets ensures accurate analyses, reproducible results, and reliable reporting.
Automating Reports and Outputs
Automation of report generation is a critical aspect of advanced clinical trials programming. Tables, listings, and graphs must be accurate, consistent, and formatted according to regulatory standards. Automating these outputs reduces human error, accelerates turnaround times, and supports reproducibility across multiple datasets or trials.
SAS macros are central to this automation. Parameterized macros can generate reports for different treatment arms, visits, or endpoints using the same underlying code structure. PROC REPORT, PROC TABULATE, and ODS GRAPHICS facilitate the creation of structured tables and high-quality visualizations. Automation also allows for quick updates when datasets change, ensuring that reports remain current without extensive reprogramming.
Integration with Statistical Analysis
Macro programming, data transformation, and workflow optimization are intrinsically linked with statistical analysis. Well-structured and validated datasets provide the foundation for accurate statistical procedures, while automated workflows streamline the generation of analysis-ready datasets.
SAS programmers collaborate closely with statisticians to translate analysis plans into executable programs. This collaboration ensures that derivations, transformations, and reporting align with study objectives, endpoints, and statistical methodologies. By integrating programming, data management, and statistical analysis, SAS programmers facilitate efficient, reliable, and auditable trial workflows.
Quality Assurance in Automated Workflows
Automation enhances efficiency but requires rigorous quality assurance to ensure reliability. Automated workflows must be tested extensively, with checks embedded to flag errors, inconsistencies, or deviations from standards.
Peer reviews, code inspections, and automated validation scripts are essential for verifying the accuracy of automated processes. Documentation of macro logic, transformation steps, and report generation procedures ensures transparency and reproducibility. These practices safeguard the integrity of data and outputs, particularly in regulated environments where audit readiness is critical.
Best Practices for Advanced Programming
Experienced SAS programmers adhere to best practices that balance efficiency, compliance, and maintainability. Key practices include:
Modular programming with reusable code components.
Parameterized macros to automate repetitive tasks while maintaining flexibility.
Comprehensive documentation and metadata for all datasets, derivations, and reports.
Rigorous validation and quality assurance procedures.
Continuous learning to stay current with SAS features, CDISC standards, and regulatory requirements.
Collaboration with statisticians, clinical teams, and data managers to ensure alignment with study objectives.
By following these practices, programmers can handle complex datasets, generate reliable outputs, and contribute to the successful execution and reporting of clinical trials.
Continuous Skill Enhancement
The field of clinical trials programming is dynamic, with evolving standards, methodologies, and software capabilities. SAS programmers must engage in continuous learning to remain proficient. This includes:
Staying updated on CDISC and regulatory guidance.
Exploring advanced SAS procedures, macros, and automation techniques.
Enhancing understanding of statistical methodologies and analysis strategies.
Adapting to new data types, including high-dimensional, longitudinal, or genomic datasets.
Continuous skill enhancement ensures that programmers can efficiently handle increasingly complex tasks, maintain compliance, and deliver high-quality, auditable outputs.
Macro programming, data transformation, validation, and workflow optimization form the backbone of advanced clinical trials programming. These techniques enable SAS programmers to efficiently manage complex datasets, automate repetitive tasks, validate results, and generate regulatory-compliant reports.
Mastery of these skills ensures accuracy, reproducibility, and audit readiness, supporting both statistical analyses and operational decision-making. By integrating advanced programming techniques with a thorough understanding of data standards and regulatory requirements, SAS programmers play a pivotal role in the successful execution and reporting of clinical trials.
Efficiency, precision, and continuous learning are the hallmarks of proficient clinical trials programmers. Through the application of advanced techniques and adherence to best practices, they contribute significantly to the integrity, transparency, and success of clinical research, ultimately supporting the development of safe and effective therapies.
Exam Preparation, Practical Strategies, and Career Outlook in Clinical Trials Programming
SAS clinical trials programming represents a specialized intersection of data management, statistical analysis, and regulatory compliance. Achieving certification as a SAS Clinical Trials Programming Professional is both a mark of technical expertise and a gateway to advanced career opportunities in the pharmaceutical and biotechnology industries.
Understanding the Certification Exam
The SAS Clinical Trials Programming Professional certification, designated as A00-282, evaluates a candidate’s proficiency in managing, analyzing, and reporting clinical trial data using SAS 9.4. The exam tests both technical knowledge and practical application of programming techniques, ensuring that certified individuals are equipped to handle the complexities of real-world clinical trials.
The examination consists of 60 to 70 questions, including multiple-choice and short-answer formats, to be completed within 110 minutes. A passing score of 68 percent is required. The questions cover a range of competencies, including data manipulation, statistical analysis, regulatory submission preparation, macro programming, and validation techniques. Familiarity with CDISC standards such as SDTM and ADaM is essential, as these frameworks underpin the organization of clinical datasets and derivation of analysis-ready outputs.
Structured Approach to Exam Preparation
Preparation for the SAS clinical trials certification requires a disciplined, systematic approach. Key strategies include:
Syllabus Review: Understanding the complete syllabus is the first step. Candidates should identify the key areas of knowledge, including clinical trial design, SAS programming fundamentals, data transformation, and reporting. This ensures focused study efforts and reduces the risk of overlooking critical topics.
Conceptual Mastery: Beyond memorizing procedures, candidates should develop a deep understanding of core concepts, including statistical analysis principles, regulatory requirements, and data standards. This conceptual knowledge allows candidates to apply techniques flexibly across diverse scenarios.
Practical Exercises: Hands-on practice is indispensable. Working with sample datasets, creating SDTM domains, generating ADaM datasets, and producing tables, listings, and graphs reinforces theoretical knowledge. Practical exercises simulate real-world challenges and improve problem-solving skills.
Sample Exams and Practice Questions: Engaging with sample exams provides insight into question formats, timing constraints, and areas that may require additional focus. These exercises help candidates build confidence and develop strategies for efficient time management during the actual exam.
Mastering SAS Programming Skills
Technical proficiency in SAS is fundamental to success. Candidates should ensure competence in:
Data Step Programming: Conditional processing, iterative loops, merging datasets, and handling missing values are core skills. Mastery of data steps ensures accurate data manipulation and transformation.
Procedures: Familiarity with procedures such as PROC SORT, PROC TRANSPOSE, PROC MEANS, PROC FREQ, PROC REPORT, and PROC TABULATE is essential. These procedures form the backbone of data analysis and reporting in clinical trials.
Macro Programming: Advanced macro skills allow for automation of repetitive tasks, dynamic report generation, and efficient handling of large datasets. Understanding macro variables, conditional logic, and iterative macro execution is critical.
Data Validation: Implementing validation checks, reconciliations, and quality assurance protocols ensures that derived datasets and outputs are accurate, compliant, and reproducible.
Proficiency in these areas not only aids exam success but also prepares candidates for real-world challenges in clinical trials programming.
Regulatory Knowledge and Data Standards
A thorough understanding of regulatory frameworks and data standards is central to clinical trials programming. Candidates must familiarize themselves with:
CDISC Standards: SDTM for raw data organization and ADaM for analysis-ready datasets. Knowledge of domain structures, controlled terminology, and metadata is critical for compliance.
Regulatory Requirements: Understanding documentation expectations, reporting formats, and validation procedures ensures that datasets and outputs meet FDA, EMA, or other regional requirements.
Traceability: Maintaining clear links between source data, derivations, and analysis outputs supports reproducibility, audit readiness, and transparency.
Integrating these principles into programming practices enhances accuracy, compliance, and professional credibility.
Effective Study Techniques
Several strategies can enhance study efficiency and knowledge retention:
Active Learning: Engaging directly with datasets, coding exercises, and practice analyses reinforces understanding more effectively than passive reading.
Incremental Practice: Breaking down study sessions into focused topics, such as SDTM mapping, ADaM derivation, or macro development, allows for gradual mastery and reduces cognitive overload.
Peer Collaboration: Participating in study groups or online forums enables discussion, clarification of complex topics, and exposure to diverse problem-solving approaches.
Review and Feedback: Regular review of practice exercises, mistakes, and feedback helps identify gaps, refine techniques, and reinforce correct procedures.
Practical Tips for Exam Success
In addition to technical preparation, practical strategies during the exam can improve performance:
Time Management: Allocate time wisely, ensuring that all questions are addressed. Avoid spending excessive time on complex questions at the expense of simpler ones.
Question Analysis: Carefully read each question to identify key requirements. Some questions test conceptual understanding, while others assess procedural knowledge.
Process Tracing: For questions involving derivations or transformations, mentally trace the steps required before executing code or selecting an answer. This reduces errors and reinforces logical thinking.
Stay Calm and Focused: Maintaining composure during the exam allows for clearer thinking and reduces mistakes caused by stress or rushing.
Career Outlook in Clinical SAS Programming
The demand for skilled SAS programmers in clinical trials continues to grow, driven by the expansion of pharmaceutical research, the globalization of clinical studies, and the increasing complexity of datasets. Clinical SAS programming offers a rewarding career with opportunities for advancement and specialization.
Key aspects of the career include:
High Demand: Pharmaceutical, biotechnology, and contract research organizations (CROs) require proficient programmers to manage and analyze clinical data.
Competitive Compensation: Clinical SAS programmers are well-compensated, reflecting the technical expertise, responsibility, and regulatory importance of the role.
Career Progression: Professionals can advance from junior SAS programmer roles to senior positions, including data manager, statistical programmer, and clinical data scientist. Leadership roles may involve overseeing programming teams, designing data workflows, or coordinating multi-center studies.
Meaningful Impact: Clinical SAS programmers contribute directly to the development of safe and effective therapies, influencing patient outcomes and public health.
Practical Applications in the Workplace
Certified clinical trials programmers apply their expertise across the full lifecycle of a trial:
Data Preparation: Cleaning, transforming, and standardizing raw datasets to ensure accuracy and compliance.
Analysis Support: Preparing analysis-ready datasets, implementing derivations, and supporting statistical evaluation.
Reporting: Generating TLFs for internal review, clinical interpretation, and regulatory submission.
Validation and QA: Implementing checks, reconciling discrepancies, and documenting processes to ensure reproducibility and regulatory compliance.
Collaboration: Working with statisticians, clinical teams, and data managers to ensure that data supports decision-making, meets study objectives, and satisfies regulatory standards.
Broader Professional Impact
Beyond individual tasks, SAS clinical trials programmers influence organizational efficiency and research integrity. Efficient data management and automation streamline workflows, reduce errors, and accelerate trial timelines. Accurate analyses and reporting ensure that decisions regarding drug efficacy, safety, and regulatory compliance are based on reliable information.
Programmers also contribute to standardization initiatives, improving interoperability across studies, organizations, and regions. By maintaining high standards of quality, reproducibility, and regulatory compliance, they reinforce the credibility and trustworthiness of clinical research as a whole.
Continuous Learning and Professional Development
The field of clinical trials programming is dynamic, with evolving standards, methodologies, and software capabilities. Continuous professional development is essential for maintaining proficiency and staying competitive. Strategies include:
Staying Current with Standards: Monitoring updates to CDISC, regulatory guidance, and best practices ensures compliance and relevancy.
Exploring Advanced SAS Features: New procedures, enhanced macro capabilities, and automation tools can improve efficiency and analytical power.
Expanding Analytical Skills: Understanding emerging statistical techniques, data visualization methods, and high-dimensional data analysis enhances versatility.
Networking and Collaboration: Engaging with peers, attending workshops, and participating in professional communities fosters knowledge exchange and exposure to diverse challenges.
Continuous learning ensures that programmers remain adaptable, capable, and prepared to tackle increasingly complex datasets, trial designs, and regulatory requirements.
Achieving SAS Clinical Trials Programming Professional certification represents a culmination of technical skill, practical experience, and domain-specific knowledge. Preparation for the certification exam requires a systematic approach, combining mastery of SAS programming, understanding of regulatory standards, proficiency in statistical analysis, and hands-on practice with clinical datasets.
Certified programmers possess the expertise to manage, analyze, and report clinical trial data accurately, efficiently, and in compliance with industry standards. They play a critical role in supporting regulatory submissions, guiding decision-making, and contributing to the development of safe and effective therapies.
The career path offers high demand, competitive compensation, opportunities for advancement, and meaningful impact on patient outcomes. Continuous learning, practical experience, and adherence to best practices ensure that SAS clinical trials programmers remain integral to the pharmaceutical and biotechnology industries.
By integrating technical competence, analytical acumen, and regulatory knowledge, certified professionals enhance the integrity, reproducibility, and efficiency of clinical research. Their work not only supports organizational goals but also contributes to the broader mission of advancing healthcare and improving patient lives.
Conclusion
The field of SAS clinical trials programming embodies a unique blend of technical expertise, analytical rigor, and regulatory compliance. Throughout the clinical trial lifecycle, programmers play a pivotal role in transforming raw data into reliable, structured, and analysis-ready datasets that support critical decision-making in pharmaceutical and biotechnology research. Mastery of clinical trial data standards, such as SDTM and ADaM, enables programmers to maintain consistency, traceability, and compliance with regulatory requirements, ensuring that datasets and outputs can withstand rigorous scrutiny. Advanced SAS programming skills, including macro development, data transformation, automation, and validation, empower professionals to efficiently manage complex datasets, generate accurate reports, and streamline workflows. Statistical analysis, quality assurance, and the production of tables, listings, and graphs further amplify the value of clinical trials programmers by providing interpretable results that inform both internal decision-making and regulatory submissions.
Preparation for the SAS Clinical Trials Programming Professional certification not only validates these competencies but also fosters practical expertise applicable in real-world trials. Certified programmers are equipped to handle intricate data challenges, collaborate effectively with statisticians and clinical teams, and contribute meaningfully to research outcomes. The career offers high demand, competitive remuneration, and growth opportunities, while also allowing professionals to impact patient care and therapeutic development. Continuous learning, adherence to best practices, and engagement with evolving standards ensure that clinical trials programmers remain indispensable in the pursuit of reliable, reproducible, and high-quality clinical research. In essence, proficiency in SAS clinical trials programming is both a technical achievement and a vital contribution to advancing healthcare.
Frequently Asked Questions
Where can I download my products after I have completed the purchase?
Your products are available immediately after you have made the payment. You can download them from your Member's Area. Right after your purchase has been confirmed, the website will transfer you to Member's Area. All you will have to do is login and download the products you have purchased to your computer.
How long will my product be valid?
All Testking products are valid for 90 days from the date of purchase. These 90 days also cover updates that may come in during this time. This includes new questions, updates and changes by our editing team and more. These updates will be automatically downloaded to computer to make sure that you get the most updated version of your exam preparation materials.
How can I renew my products after the expiry date? Or do I need to purchase it again?
When your product expires after the 90 days, you don't need to purchase it again. Instead, you should head to your Member's Area, where there is an option of renewing your products with a 30% discount.
Please keep in mind that you need to renew your product to continue using it after the expiry date.
How often do you update the questions?
Testking strives to provide you with the latest questions in every exam pool. Therefore, updates in our exams/questions will depend on the changes provided by original vendors. We update our products as soon as we know of the change introduced, and have it confirmed by our team of experts.
How many computers I can download Testking software on?
You can download your Testking products on the maximum number of 2 (two) computers/devices. To use the software on more than 2 machines, you need to purchase an additional subscription which can be easily done on the website. Please email support@testking.com if you need to use more than 5 (five) computers.
What operating systems are supported by your Testing Engine software?
Our testing engine is supported by all modern Windows editions, Android and iPhone/iPad versions. Mac and IOS versions of the software are now being developed. Please stay tuned for updates if you're interested in Mac and IOS versions of Testking software.


