Certification: Application Consultant
Certification Full Name: Application Consultant, Implementation Consultant, Solution Consultant, Business Process Consultant
Certification Provider: SAP
Exam Code: C_TPLM40_65
Exam Name: SAP Certified Application Associate - Quality Management with SAP ERP 6.0 EHP5
Product Screenshots
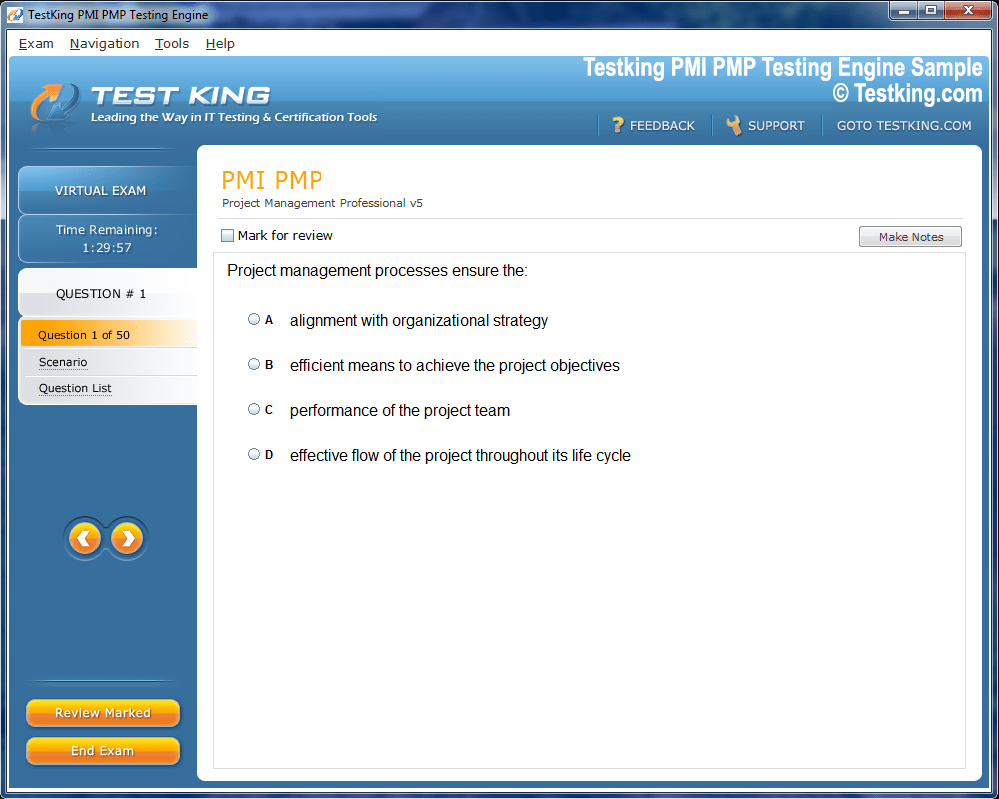
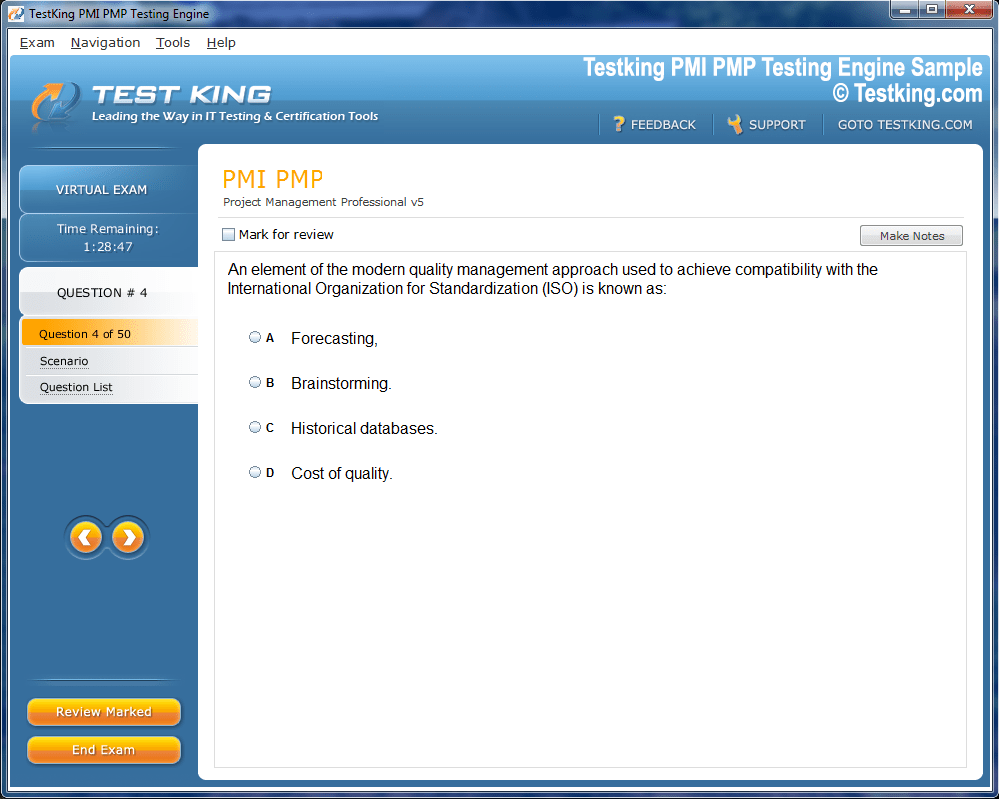
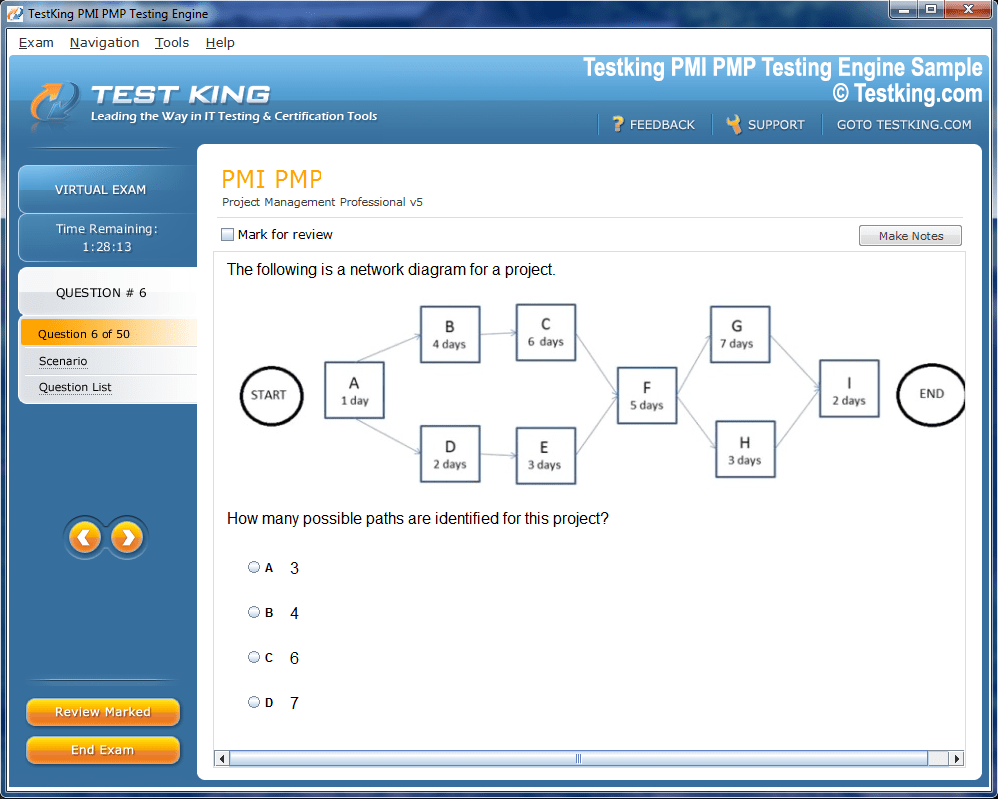
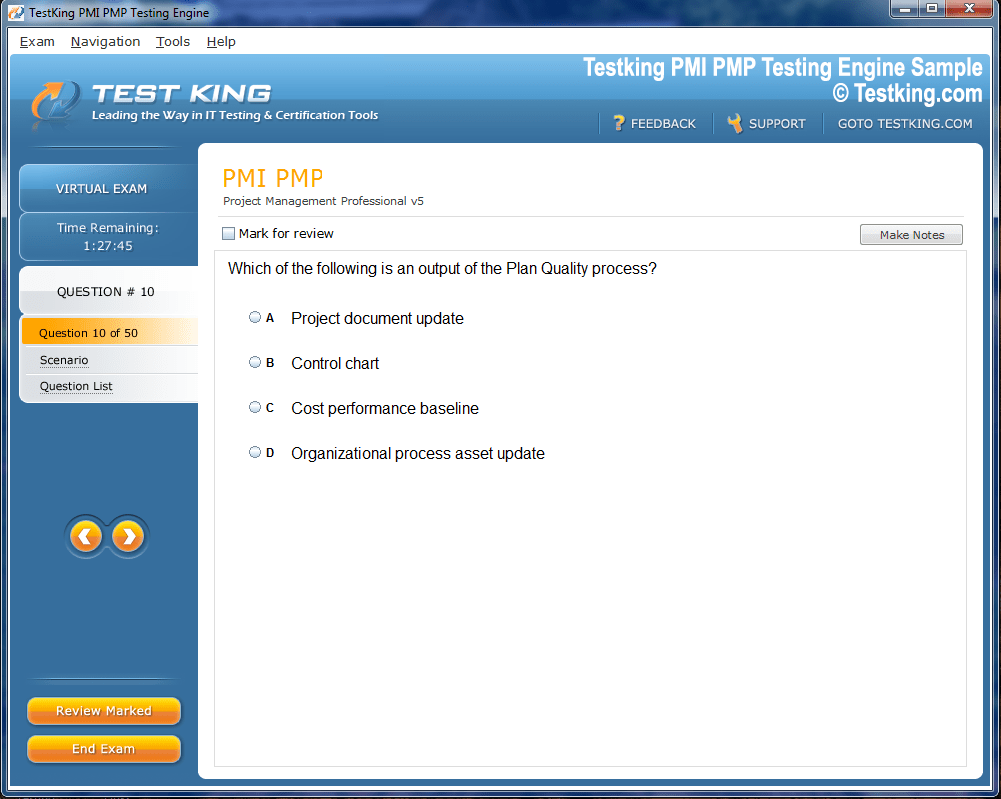
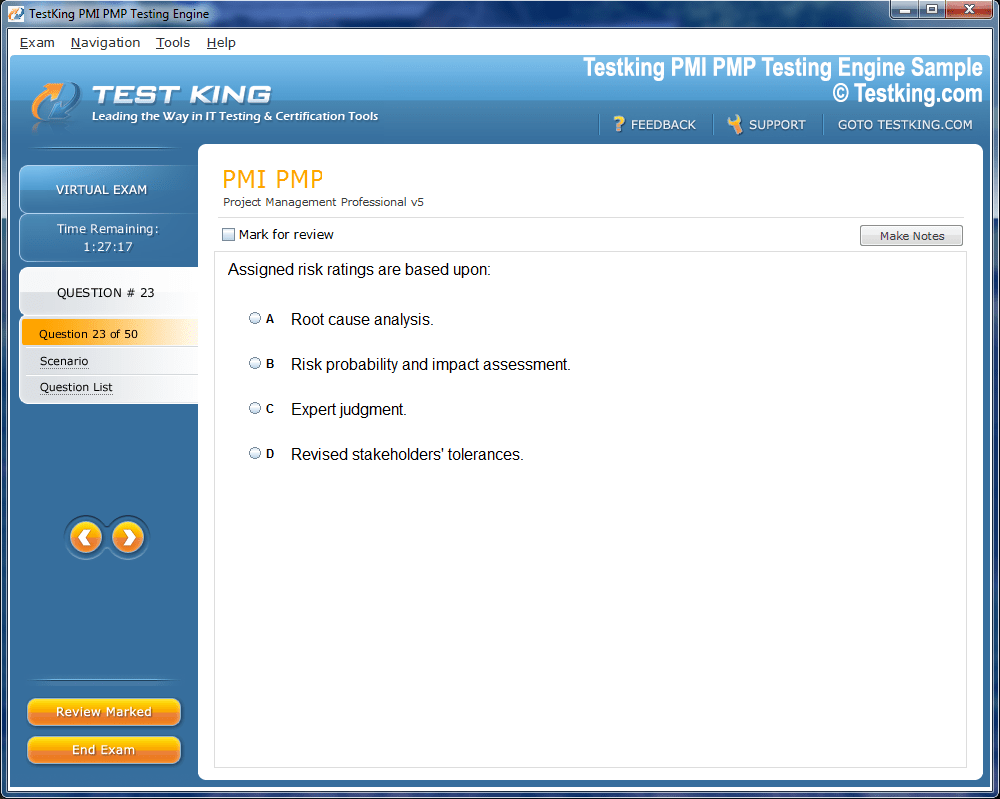
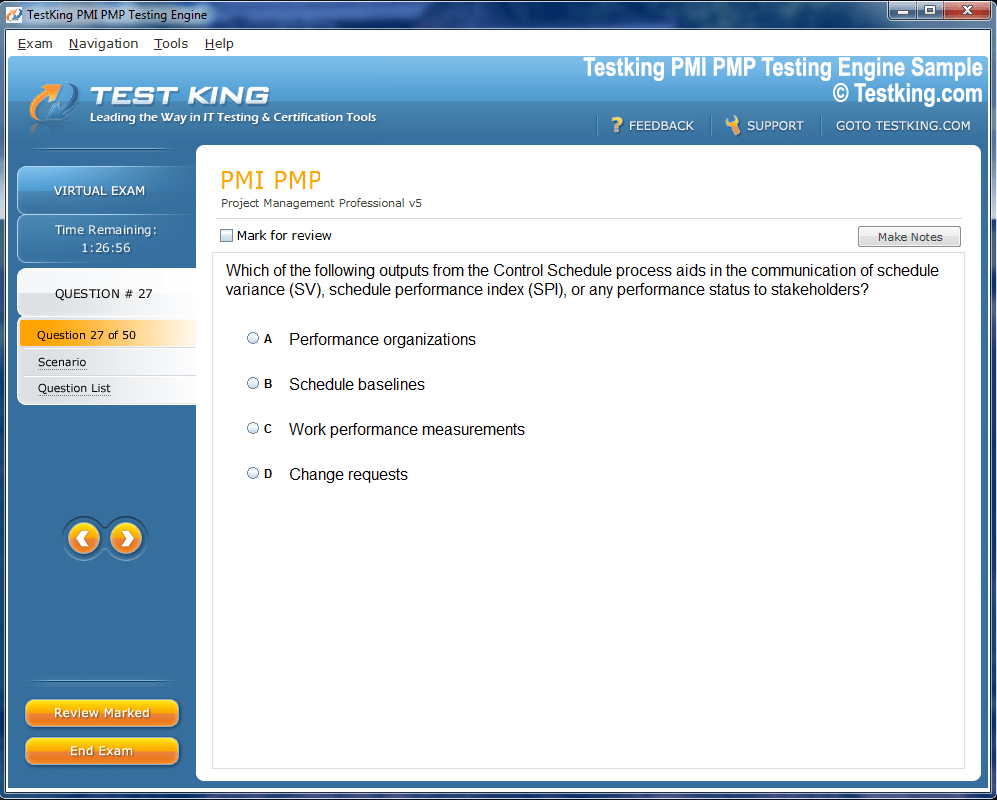
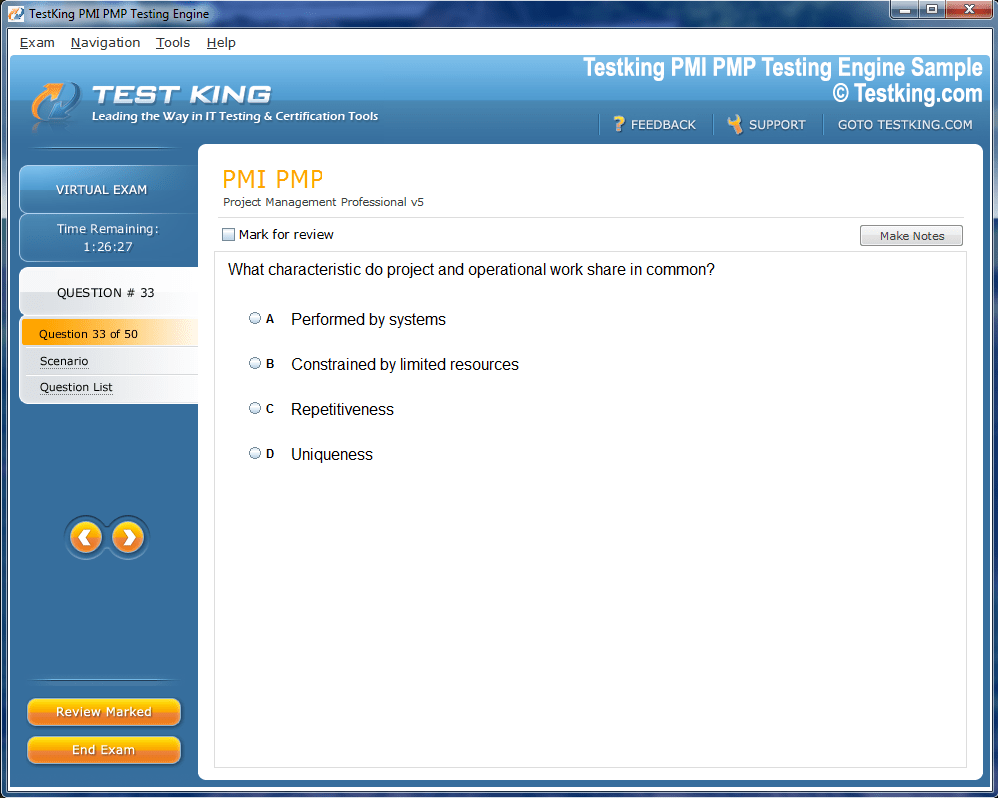
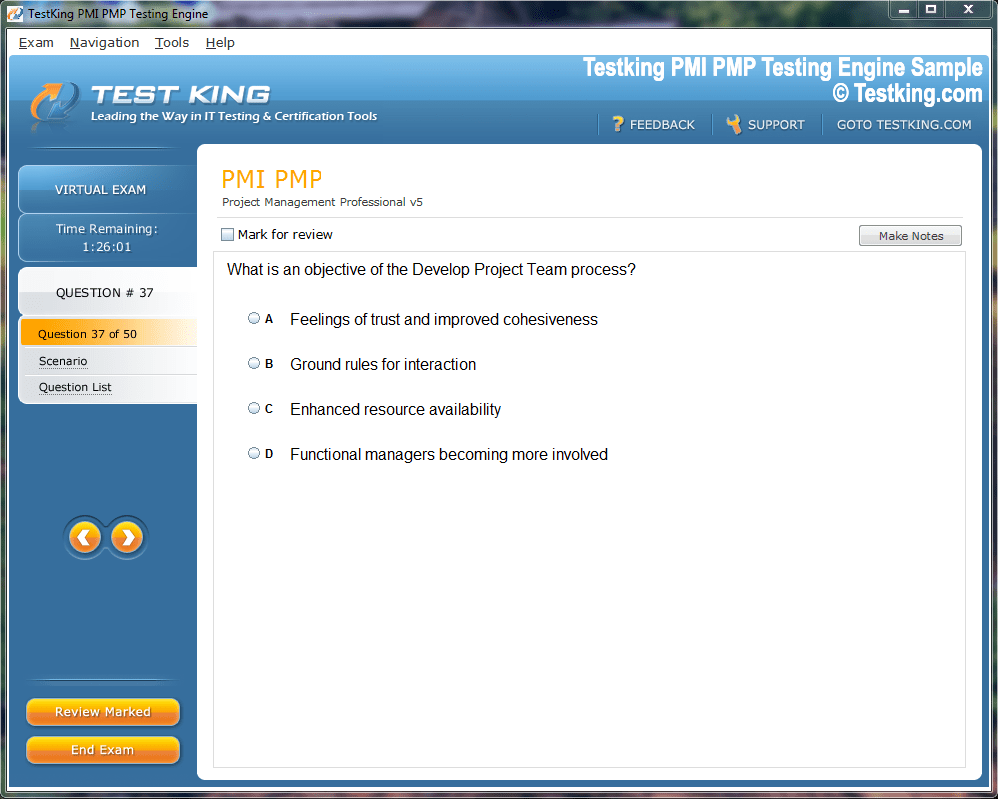
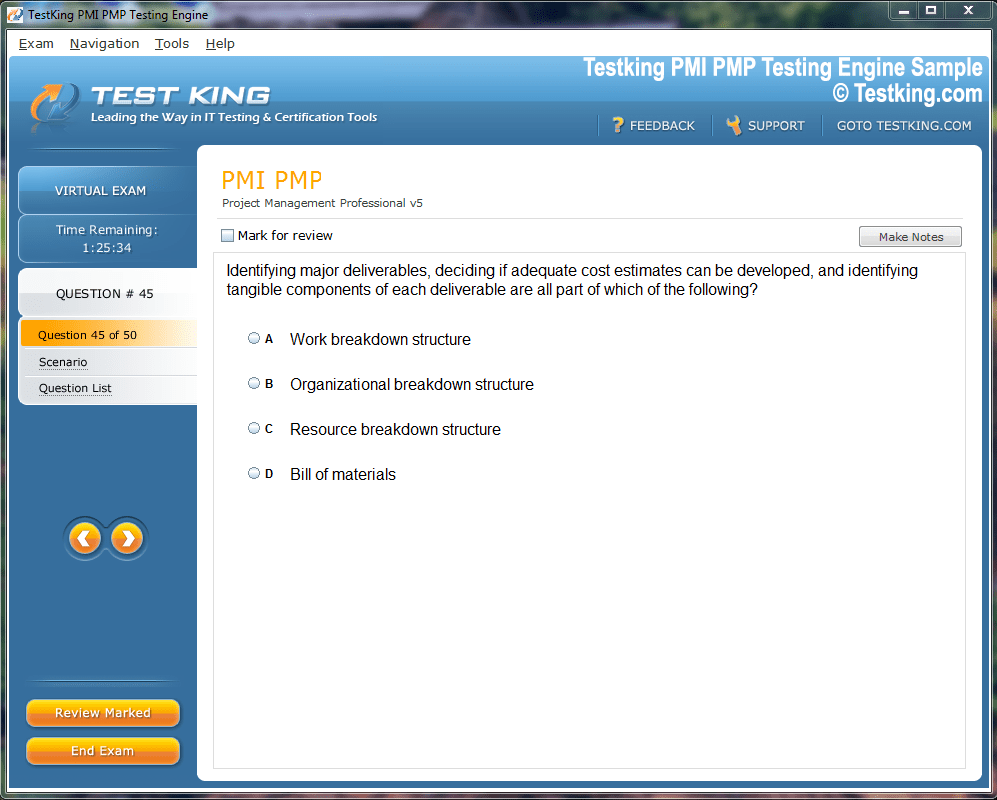
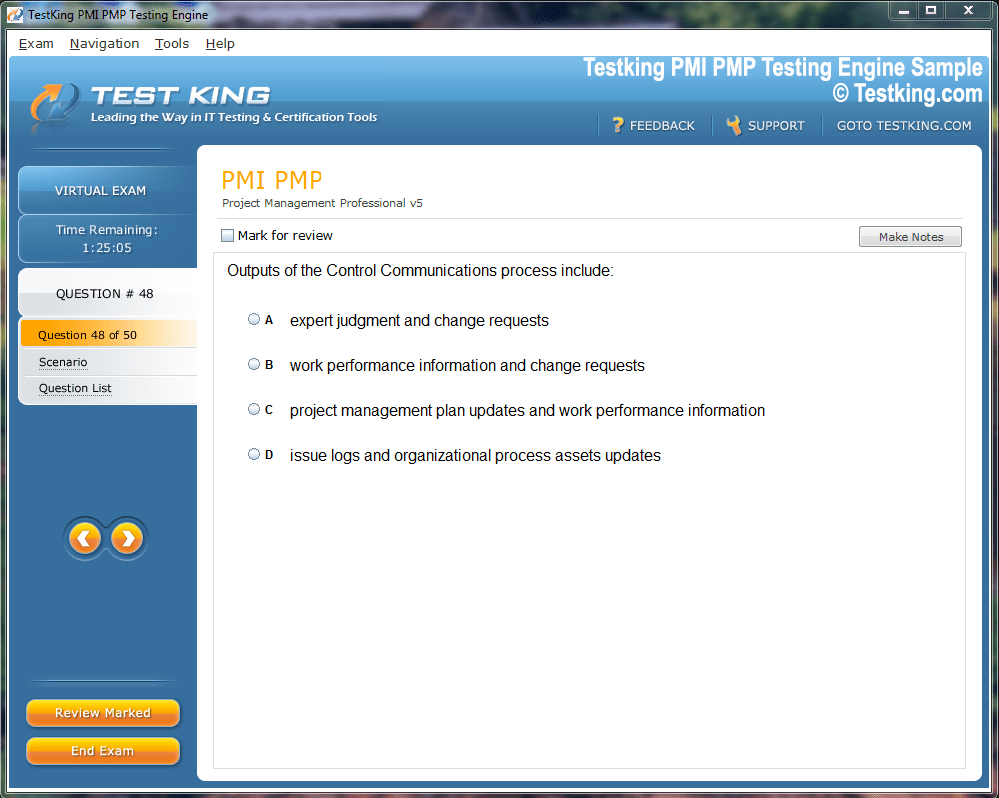
nop-1e =1
A Complete Guide to SAP C_TPLM40_65 Certification Success
The SAP C_TPLM40_65 certification, officially recognized as SAP Certified Application Associate – Quality Management with SAP ERP 6.0 EhP5, serves as an essential qualification for professionals aspiring to master the fundamental dimensions of SAP Quality Management. This credential validates not only a candidate’s theoretical knowledge but also the ability to translate concepts into practical applications under the supervision of seasoned consultants. It is a gateway for anyone eager to explore the intricate structures and operational subtleties of Quality Management within the SAP environment.
SAP Quality Management, or QM, encompasses a comprehensive suite of tools and processes designed to sustain superior quality levels across a company’s supply chain and production ecosystem. By earning the C_TPLM40_65 certification, individuals demonstrate that they have internalized the basic framework of SAP QM and can proficiently maneuver through the complexities that arise during project execution. This recognition extends beyond a simple examination; it reflects a person’s commitment to rigorous preparation and an ability to integrate quality assurance principles into diverse organizational contexts.
Significance of Entry-Level Accreditation
The C_TPLM40_65 certification is particularly significant because it is structured as an entry-level qualification, allowing a broad spectrum of candidates to engage with SAP Quality Management without requiring extensive prior experience. This inclusive nature makes it ideal for professionals transitioning into SAP roles or those seeking to reinforce their foundational knowledge of quality assurance within an ERP system.
Entry-level certifications carry a distinct gravitas in professional landscapes because they provide a structured, verifiable route to demonstrate aptitude. They establish a fundamental understanding that can later be built upon with advanced learning and specialized roles. The C_TPLM40_65 exam exemplifies this philosophy by focusing on the critical building blocks of Quality Management, ensuring that candidates develop a holistic perspective rather than a fragmented or superficial grasp.
Candidates who secure this credential signal to employers and project leaders that they possess not only theoretical insights but also the capacity to participate actively in real-world implementations. By fostering familiarity with core SAP QM processes, this certification cultivates confidence and competence, both of which are indispensable when collaborating on multifaceted projects or integrating quality control mechanisms into production environments.
Key Themes and Conceptual Landscape
At the heart of the C_TPLM40_65 certification lies an intricate syllabus designed to evaluate a candidate’s grasp of numerous essential domains. These areas encompass sample management, quality certificates, quality notifications, audit management, configuration and organization, as well as the integration of QM with other SAP modules like Materials Management and Sales & Distribution. The curriculum extends to process industries, discrete manufacturing, and the implementation and configuration of quality inspections, planning, and control.
Sample management, for instance, requires an understanding of how materials are inspected and verified through methodical sampling techniques. Quality certificates involve meticulous documentation that validates product conformity with specified standards. Quality notifications and audit management emphasize the importance of identifying nonconformities, tracking incidents, and ensuring continuous improvement through systematic review processes.
Equally significant is the integration of QM with other SAP components such as MM and S&D. This interconnectedness reflects real organizational dynamics, where quality considerations permeate procurement, sales, and service functions. A candidate must be able to conceptualize how quality measures influence and harmonize with broader enterprise workflows, ensuring that no process remains siloed.
The Preparatory Journey
Preparation for the C_TPLM40_65 examination demands a judicious blend of strategy, discipline, and intellectual curiosity. Candidates benefit from formal SAP training, which offers structured exposure to the material and provides nuanced explanations of complex concepts. While self-study remains valuable, guided instruction often accelerates comprehension and helps to clarify ambiguous areas that might otherwise lead to confusion.
A comprehensive study plan is indispensable. Aspiring professionals should craft a schedule that systematically covers each segment of the syllabus, ensuring no topic is overlooked. Because each domain carries a relatively equal weight in the examination, selective studying can be detrimental. Instead, candidates should cultivate an even-handed approach, allocating sufficient time to master every theme thoroughly.
Breaks and rest periods are integral to this preparation. Sustained mental exertion without pause often leads to diminishing returns, as cognitive fatigue impairs retention and analytical ability. Regular intervals of repose help refresh the mind, enabling more effective study sessions. Integrating such practices into a structured timetable reinforces focus and sharpens critical thinking.
Equally important is the practice of note-taking. Capturing key ideas, terminologies, and procedural steps in an organized manner serves as an invaluable resource for later revision. These notes become a personalized compendium of insights, streamlining the review process and highlighting connections that might not be immediately apparent during initial study.
Evaluating Competence Through Practice
Self-assessment is another pivotal component of successful preparation. Practice tests provide an authentic simulation of the actual examination environment, allowing candidates to gauge their readiness while identifying areas that require further attention. These mock exams replicate the timing and question formats of the official test, offering a practical benchmark for progress.
Consistent practice instills familiarity with the exam’s rhythm and complexity, reducing anxiety and enhancing performance. It also reveals subtle weaknesses—perhaps in topics such as audit management or integration with S&D—that might otherwise remain concealed until the day of the test. By confronting these deficiencies early, candidates can reinforce their knowledge and approach the examination with confidence.
While some might be tempted to rely on unauthorized dumps or shortcut methods, such strategies often prove counterproductive. They bypass the deep learning that underpins long-term competence and may leave candidates ill-equipped to apply their knowledge in practical settings. Genuine practice and deliberate study cultivate not only exam readiness but also the ability to navigate real SAP environments with dexterity.
Exploring SAP Quality Management in Depth
Understanding the broader context of SAP Quality Management enriches the significance of the C_TPLM40_65 certification. SAP QM functions as a cornerstone of the SAP Logistics framework, supporting the planning, supervision, and evaluation of quality across internal processes, external partnerships, inbound supply chains, and outbound deliveries. It is a multifaceted system that facilitates rigorous quality oversight from inception to completion.
Within this framework, organizations can schedule and orchestrate inspections and tests at critical junctures, ensuring that every stage of production aligns with established quality benchmarks. This meticulous oversight accelerates positive releases, minimizes errors, and enables the rapid generation of quality reports and certificates. The system’s integrative capabilities foster a seamless flow of information, allowing stakeholders to respond swiftly to discrepancies and maintain unwavering standards.
The advantages of SAP QM extend into several pivotal domains. Its collaboration with other SAP modules, such as Production Planning and Materials Management, exemplifies how quality management permeates diverse operational layers. The system allows enterprises to maintain consistent data related to customers, suppliers, and vendors, ensuring that quality considerations remain visible and actionable across the entire organizational spectrum.
Strategic Benefits for Organizations
Implementing SAP QM yields a multitude of benefits. Standardized quality processes enable organizations to monitor procedures meticulously, identify anomalies, and issue quality notifications when deviations occur. Automated mechanisms, such as the creation of inspection lots upon production order release or goods receipt, further enhance efficiency and accuracy.
The system supports comprehensive quality compliance, extending from the evaluation of raw materials to in-process manufacturing checks and final product inspections. This end-to-end oversight safeguards product integrity and bolsters customer satisfaction. Additionally, SAP QM facilitates compliance with specific client requirements, ensuring that goods shipped to customers align with purchase agreements and quality expectations.
Source inspections represent another critical capability. Through SAP QM, company representatives can visit supplier facilities to assess the quality of goods at their origin, ensuring adherence to established standards before materials enter the production pipeline. Routine quality inspections upon delivery further reinforce this vigilant approach, as inspection lots are automatically created for incoming goods linked to purchase orders.
Professional and Organizational Impact
For individuals, obtaining the C_TPLM40_65 certification opens doors to new professional opportunities and enhances credibility in the competitive field of SAP consulting. It signals to employers that the candidate is proficient in foundational Quality Management concepts and prepared to contribute effectively to projects requiring precise quality control measures.
For organizations, employing certified professionals means gaining team members capable of implementing SAP Product Lifecycle Management and Quality Management solutions with confidence. This expertise directly influences operational performance, enabling enterprises to maintain stringent quality standards, optimize processes, and achieve strategic objectives with greater consistency.
Integration of SAP Quality Management with Core Modules
SAP Quality Management functions most effectively when integrated with other key SAP modules, creating a cohesive ecosystem that enables organizations to monitor and enhance quality throughout their processes. One of the most prominent connections exists between QM and Materials Management, where inspection lots, vendor evaluations, and material movements are synchronized to maintain consistency and accuracy. This integration allows for automatic generation of inspection tasks whenever a goods receipt or production order is processed, ensuring that no step in the supply chain bypasses quality scrutiny.
Beyond Materials Management, SAP QM also integrates with Sales and Distribution (S&D) and Service modules. In this context, quality checks extend to customer-facing processes, ensuring that deliveries conform to contractual agreements and service standards. By embedding quality management into both procurement and distribution, organizations can achieve a holistic view of their operations, detecting discrepancies early and minimizing the risk of nonconformance affecting downstream processes.
Understanding Core Syllabus Components
The C_TPLM40_65 exam syllabus emphasizes several pivotal areas that candidates must master. Sample management is a foundational concept, involving systematic inspection of materials to ensure adherence to specifications. Candidates must understand sampling techniques, lot sizes, and statistical quality control methods to apply this knowledge effectively.
Quality certificates represent another crucial element. They document compliance with predefined quality standards and are often required for regulatory or client purposes. Candidates must be able to generate, interpret, and manage these certificates within the SAP system to verify material integrity and process adherence.
Quality notifications and audit management focus on identifying deviations and implementing corrective actions. Notifications capture nonconformities, while audit management involves systematic reviews to ensure adherence to quality processes. Mastery of these areas allows professionals to monitor compliance, document discrepancies, and facilitate continuous improvement initiatives.
The syllabus further includes implementation and configuration of quality inspection, planning, and control. These topics require a practical understanding of how to define inspection plans, create control mechanisms, and execute quality checks within both discrete manufacturing and process industries. Successful integration of these components ensures a robust quality management system capable of addressing diverse operational scenarios.
Structured Study Approaches
Effective preparation for the C_TPLM40_65 certification relies on a structured study approach. Creating a detailed schedule allows candidates to allocate time efficiently, ensuring comprehensive coverage of all syllabus topics. This schedule should balance theoretical learning with hands-on practice, enabling a deeper understanding of SAP QM functionalities.
Regular breaks are critical to maintain cognitive efficiency. Studying for prolonged periods without rest often leads to mental fatigue and diminished retention. Short, strategic pauses between study sessions allow the mind to consolidate information and maintain focus during subsequent study periods.
Note-taking is another essential strategy. Recording key concepts, procedures, and configurations in a structured manner creates a valuable reference for revision. These notes can highlight interconnections between topics and reinforce understanding, providing a personalized guide that supports long-term retention and practical application.
Practice and Simulation
Practice tests are integral to exam readiness. They simulate the structure, timing, and difficulty level of the official C_TPLM40_65 examination, allowing candidates to assess their knowledge and identify areas requiring further focus. By repeatedly practicing under exam-like conditions, candidates can reduce anxiety and improve both speed and accuracy.
Self-assessment through practice also reveals weaknesses that might not be apparent during theoretical study. Areas such as integration with S&D or audit management may require additional attention to achieve proficiency. Continuous evaluation ensures that candidates approach the examination with confidence, equipped with a well-rounded understanding of all syllabus domains.
Avoiding shortcuts is vital for sustainable competence. Relying on unauthorized dumps or incomplete study methods may lead to temporary success, but fails to cultivate practical skills. Comprehensive preparation, including practice, conceptual understanding, and hands-on experience, ensures that knowledge can be effectively applied in real-world SAP QM scenarios.
SAP Quality Management Functionality
SAP QM provides extensive functionality to support quality oversight across all phases of production and supply chain operations. The module enables scheduling, monitoring, and documentation of quality checks at critical points, facilitating proactive quality control and continuous improvement. This functionality encompasses planning inspections, tracking inspection results, and generating certificates that validate adherence to internal and external standards.
Integration with other modules enhances the utility of SAP QM. For example, inspection lots generated during goods receipt in Materials Management ensure that incoming materials meet quality requirements before they enter production. Similarly, integrating quality checks with Production Planning allows real-time monitoring of process compliance, reducing the likelihood of defects and minimizing wastage.
SAP QM also supports regulatory compliance and customer-specific requirements. By maintaining a detailed record of quality checks and inspection results, organizations can demonstrate adherence to contractual and statutory obligations. This capability is especially important in industries where product integrity and safety are critical, such as pharmaceuticals, automotive, and food processing.
Organizational Advantages
The deployment of SAP QM yields significant organizational advantages. Standardized processes enable consistent quality monitoring, defect detection, and corrective action management. Automated workflows, such as the creation of inspection lots and quality notifications, reduce manual intervention and improve operational efficiency.
By ensuring quality at every stage—from procurement to production to delivery—SAP QM strengthens overall operational resilience. Organizations can minimize nonconformances, enhance customer satisfaction, and maintain a reputation for reliability and excellence. Data maintained within the system provides actionable insights, enabling informed decision-making and continuous process improvement.
Source inspections exemplify the proactive quality approach enabled by SAP QM. Representatives can visit supplier facilities to assess material quality before shipment, ensuring that upstream processes do not compromise final product integrity. Similarly, inspection lots for received goods automatically trigger upon delivery, providing immediate quality validation and preventing defective materials from entering production.
Professional Growth and Certification Impact
For individuals, the C_TPLM40_65 certification signifies mastery of foundational SAP Quality Management concepts. It demonstrates the ability to participate in project implementations, configure inspection plans, and manage quality processes with competence. Certification holders are recognized for their technical proficiency and capacity to contribute effectively to quality management initiatives.
From an organizational perspective, employing certified professionals enhances overall capability. Teams gain members capable of implementing and maintaining SAP QM processes with precision, ensuring adherence to quality standards and supporting continuous improvement. This expertise translates into better operational outcomes, improved compliance, and increased efficiency across the enterprise.
Mastering Quality Planning and Control
Quality planning within SAP QM involves defining inspection methods, sampling procedures, and inspection criteria to ensure consistency and compliance. Candidates must understand how to create inspection plans tailored to both discrete manufacturing and process industries. These plans serve as the blueprint for quality monitoring, guiding inspection activities, and providing measurable benchmarks for evaluation.
Quality control, on the other hand, focuses on executing inspection plans and analyzing results. Effective control mechanisms allow organizations to detect deviations, implement corrective actions, and prevent recurrence. By integrating quality control with other operational modules, enterprises achieve a continuous feedback loop that informs process improvements and enhances product reliability.
Continuous Improvement through SAP QM
SAP Quality Management is inherently aligned with continuous improvement principles. The module’s capabilities for monitoring, auditing, and reporting enable organizations to identify inefficiencies, track defects, and implement corrective measures systematically. Quality notifications document nonconformities and facilitate structured follow-up, ensuring that improvement initiatives are data-driven and results-oriented.
The ability to generate detailed reports and certificates enhances transparency and accountability. Stakeholders can access information about inspection outcomes, corrective actions, and compliance status, fostering a culture of responsibility and excellence. By leveraging these insights, organizations can refine processes, enhance product quality, and sustain competitive advantage in increasingly demanding markets.
The Role of Sample Management in SAP Quality Management
Sample management is one of the foundational elements within SAP Quality Management, playing a pivotal role in ensuring that materials meet predefined quality standards before they enter production or distribution. The concept revolves around selecting representative samples from larger batches and inspecting them against specified criteria. This process allows organizations to make informed decisions about material acceptance, mitigating risks associated with defective inputs.
Candidates preparing for the C_TPLM40_65 certification must understand various sampling techniques, including random sampling, stratified sampling, and systematic sampling. Each technique is selected based on the nature of the material, production processes, and desired confidence levels. SAP QM facilitates the creation of sampling procedures that are linked to inspection plans, ensuring that quality checks are consistent, repeatable, and auditable.
Inspection lots generated in SAP QM for sample management are tracked meticulously within the system. These lots provide structured frameworks for recording inspection results, assigning responsibility, and managing follow-up actions. Mastery of sample management concepts equips professionals to ensure that production inputs are consistently validated, reducing the likelihood of downstream quality issues and enhancing overall operational reliability.
Quality Certificates and Compliance Documentation
Quality certificates are an essential component of SAP Quality Management, serving as formal documentation that materials or products meet established standards. These certificates are used both internally and externally to verify compliance with regulatory requirements, client specifications, and organizational policies. They reflect an organization’s commitment to quality and provide traceability in the event of audits or inspections.
Within the SAP environment, candidates must be adept at generating, managing, and interpreting quality certificates. This involves understanding the underlying data structures, configuring certificate types, and ensuring accurate linkage to inspection lots. Quality certificates also facilitate seamless communication with suppliers and customers, providing verified proof that materials meet contractual and statutory expectations.
For professionals, the ability to handle quality certificates effectively is crucial. It allows them to maintain operational transparency, streamline reporting processes, and support compliance initiatives. Integrating quality certificates with other QM processes, such as audit management and inspection planning, enhances the robustness of the overall quality framework.
Quality Notifications and Audit Management
Quality notifications are a mechanism within SAP QM to capture, track, and resolve nonconformities. When a defect is detected during inspection, production, or post-delivery, a quality notification is created to document the issue and trigger corrective actions. Candidates must understand the different types of notifications, their workflow, and how they integrate with other modules to ensure timely resolution and continuous improvement.
Audit management complements this process by systematically evaluating quality processes and identifying areas for enhancement. Audits provide structured assessments, documenting compliance with internal and external standards. Through audit management, organizations can detect systemic issues, implement corrective measures, and maintain a culture of accountability and precision.
For C_TPLM40_65 aspirants, proficiency in both quality notifications and audit management demonstrates the ability to manage deviations effectively. It ensures that candidates can contribute to maintaining high-quality standards, reducing defects, and supporting the organization’s broader quality assurance objectives.
Integration of QM with Materials Management and Production Planning
SAP QM’s integration with Materials Management (MM) and Production Planning (PP) is vital for maintaining a seamless quality ecosystem. In MM, inspection lots for received materials are automatically generated during goods receipt, ensuring that only compliant materials enter production. This integration minimizes risks associated with defective inputs, streamlines procurement workflows, and ensures adherence to supplier agreements.
In Production Planning, quality management processes ensure that inspections occur at critical stages of manufacturing. Inspection plans, control strategies, and sampling procedures are aligned with production schedules, enabling real-time monitoring of quality compliance. Candidates must understand how these integrations function, including the configuration of inspection types, quality levels, and notification triggers.
The synergy between QM, MM, and PP allows organizations to create a continuous feedback loop. Deviations detected during production can trigger immediate corrective actions, supplier notifications, or process adjustments. This integrated approach reinforces the principle of proactive quality management, ensuring consistency and reliability across operations.
Implementing Quality Inspection and Planning
Quality inspection and planning are central to SAP QM, providing the mechanisms to evaluate products, materials, and processes systematically. Inspection planning involves defining the sequence of checks, methods, and sampling criteria, while execution ensures that inspections are conducted accurately and results are recorded appropriately.
C_TPLM40_65 candidates must understand how to configure inspection types, link them to material master records, and define characteristics for measurement. The inspection process may involve manual or automated steps, depending on production complexity. Mastery of inspection planning enables professionals to anticipate quality risks, structure inspections efficiently, and maintain compliance with internal and external standards.
Quality planning extends beyond individual inspections to encompass entire production cycles. By establishing control strategies, inspection plans, and sampling procedures, organizations can monitor product quality consistently throughout the manufacturing process. This holistic perspective ensures that defects are detected early, corrective actions are implemented promptly, and overall production efficiency is enhanced.
Quality Control and Discrete Manufacturing
Quality control in SAP QM involves applying inspection results to make informed decisions about material acceptance, rework, or rejection. Candidates must understand how to analyze inspection data, interpret results, and trigger notifications or corrective actions when deviations occur. Effective quality control reduces waste, enhances product reliability, and ensures compliance with customer and regulatory requirements.
In discrete manufacturing, quality control plays a crucial role in maintaining production integrity. Products are inspected at defined stages, with inspection results influencing subsequent process steps. C_TPLM40_65 aspirants must understand how to configure quality control procedures, manage inspection lots, and integrate control activities with production orders. This knowledge allows professionals to implement robust quality processes, ensuring consistency and minimizing defects.
Discrete manufacturing scenarios often involve complex production lines, multiple inspection points, and diverse materials. SAP QM’s functionalities enable detailed tracking, reporting, and analysis of inspection outcomes, providing actionable insights that support continuous improvement. Mastery of these processes ensures that candidates can contribute effectively to quality management initiatives in dynamic manufacturing environments.
SAP QM in Process Industries
Process industries, such as chemicals, pharmaceuticals, and food production, present unique quality challenges due to continuous production flows and stringent regulatory requirements. SAP QM addresses these complexities by enabling real-time monitoring, batch management, and comprehensive documentation of inspection results.
Candidates must understand how to configure inspection plans for process industries, including defining characteristics, sampling procedures, and control strategies. Batch management ensures traceability, allowing organizations to link quality inspections to specific production lots. This capability is essential for compliance, recall management, and ensuring consistent product quality.
SAP QM also supports integration with laboratory management and analytics tools, enabling precise measurement and evaluation of critical quality attributes. Professionals equipped with these skills can help organizations maintain operational integrity, comply with regulatory standards, and implement continuous improvement initiatives in highly regulated environments.
Benefits of SAP QM in Organizational Contexts
SAP Quality Management provides multiple organizational advantages. Standardized processes enable consistent quality monitoring, defect detection, and corrective action management. Automated workflows reduce manual intervention, streamline reporting, and enhance operational efficiency.
Integration with procurement, production, and distribution ensures that quality considerations are embedded across all operational layers. This reduces the risk of defective products reaching customers, strengthens supplier accountability, and enhances overall supply chain reliability. By maintaining detailed records of inspections, notifications, and audits, organizations can demonstrate compliance and foster a culture of accountability and continuous improvement.
Source inspections exemplify SAP QM’s proactive approach. Representatives can assess supplier quality at the origin, preventing nonconforming materials from entering production. Inspection lots for delivered goods are generated automatically, facilitating immediate validation and corrective actions. These capabilities ensure that quality management is not reactive but embedded into everyday operations.
Professional Impact of Certification
The C_TPLM40_65 certification enhances both individual and organizational competence. For professionals, it signifies mastery of foundational SAP QM principles, including inspection planning, quality control, notifications, and integration with other modules. Certified individuals are equipped to contribute effectively to projects, implement robust quality processes, and support continuous improvement initiatives.
For organizations, employing certified professionals ensures that quality management processes are implemented accurately and efficiently. Teams gain the ability to monitor compliance, reduce defects, and enhance operational performance. Certification validates the expertise of personnel, fostering confidence in the organization’s ability to maintain high-quality standards and meet customer expectations.
Continuous Improvement and Long-Term Benefits
SAP QM is designed to support continuous improvement initiatives. Through systematic inspections, notifications, audits, and reporting, organizations can identify inefficiencies, implement corrective actions, and monitor progress over time. The module’s integration with production, materials management, and distribution ensures that improvements are applied consistently across operations.
C_TPLM40_65 candidates trained in continuous improvement principles can contribute significantly to organizational quality initiatives. By leveraging SAP QM functionalities, professionals help ensure operational consistency, reduce waste, and enhance customer satisfaction. These long-term benefits extend beyond immediate project needs, creating enduring value for both individuals and enterprises.
Implementing Quality Management in Real-World Projects
SAP Quality Management is most effective when its principles are applied to real-world organizational projects. Implementation requires a blend of technical knowledge, procedural understanding, and practical problem-solving skills. Candidates pursuing the C_TPLM40_65 certification must be adept at configuring SAP QM modules to align with business processes, ensuring that quality oversight is both rigorous and seamless.
Successful implementation begins with a thorough analysis of existing processes, identifying areas where quality interventions are needed. This involves mapping procurement, production, and distribution workflows and determining how inspection plans, notifications, and audits can be integrated. By establishing clear procedures for monitoring quality at critical stages, organizations can detect deviations early, reduce defects, and maintain compliance with internal and external standards.
Configuring Inspection Plans and Procedures
Inspection plans are the blueprint of SAP QM, dictating how quality checks are conducted. Candidates must understand how to configure inspection types, link them to material masters, and define characteristics that need to be measured. These plans ensure that inspections are standardized, repeatable, and auditable.
Inspection procedures must also accommodate the nuances of both discrete and process industries. In discrete manufacturing, inspections often occur at specific points along a production line, while in process industries, continuous monitoring may be necessary. SAP QM allows for flexible configuration to suit these varied requirements, ensuring that inspections are relevant, efficient, and effective.
Mastery of inspection planning ensures that candidates can design processes that prevent defects, streamline production, and maintain high levels of operational quality. This competency is a cornerstone of both certification success and practical application in professional environments.
Managing Quality Notifications
Quality notifications are central to SAP QM’s approach to issue resolution. When defects or deviations are detected, notifications are generated to document the problem and trigger corrective action. Candidates must understand the lifecycle of a notification, including creation, classification, assignment, resolution, and closure.
Notifications are integrated with other modules, ensuring that problems detected in procurement, production, or delivery are communicated across the relevant teams. For example, a defect detected in a supplier shipment can trigger a notification that alerts procurement, triggers an audit, and initiates corrective measures. Mastery of this workflow allows candidates to manage quality issues proactively, ensuring that they are addressed before they escalate into larger problems.
Conducting Audits for Continuous Improvement
Audits within SAP QM provide a systematic evaluation of quality processes and ensure that they align with organizational standards. Candidates must understand how to plan, schedule, and execute audits, as well as how to record findings and recommend corrective actions. Audits serve as both preventive and corrective tools, identifying weaknesses before they impact operations and ensuring that improvements are sustained over time.
Incorporating audit management into quality processes also facilitates regulatory compliance. Organizations can document adherence to industry standards, track deviations, and demonstrate accountability during inspections. This capability is particularly valuable in industries such as pharmaceuticals, food production, and automotive, where strict quality regulations are enforced.
Integration with Supplier and Customer Processes
SAP QM extends its influence beyond internal operations to encompass supplier and customer interactions. Source inspections allow organizations to evaluate supplier quality at the point of origin, ensuring that materials meet specifications before entering the production process. This proactive approach reduces defects and enhances supply chain reliability.
Similarly, quality checks in the delivery process ensure that products conform to customer expectations and contractual agreements. Inspection results, notifications, and certificates are shared as part of transparent communication, enhancing trust and accountability. Candidates must understand these integrations to effectively manage quality across the entire value chain.
Benefits of Automation in SAP QM
Automation is a key advantage of SAP QM, enabling organizations to execute quality processes efficiently and consistently. Automated inspection lot creation, for instance, reduces manual errors and ensures that inspections occur without delays. Similarly, automated reporting, notifications, and certificate generation streamline workflow and enhance operational visibility.
Candidates must be adept at configuring automated processes within SAP QM to achieve maximum efficiency. Understanding the triggers, dependencies, and outcomes of automation allows professionals to design workflows that reduce manual intervention, accelerate decision-making, and maintain consistent quality standards across all operations.
Quality Management in Process Industries
Process industries present unique challenges due to continuous production flows, complex materials, and strict regulatory requirements. SAP QM supports these industries by enabling batch management, real-time monitoring, and precise documentation of inspection results. Candidates must understand how to configure inspection plans for process industries, define critical characteristics, and manage batch traceability.
Batch traceability ensures that quality issues can be traced back to specific production lots, facilitating recalls, corrective actions, and regulatory reporting. By mastering these functionalities, candidates demonstrate the ability to maintain operational integrity and compliance in environments where product quality and safety are critical.
Leveraging SAP QM for Data-Driven Decisions
One of the most significant advantages of SAP QM is its ability to generate actionable insights through data analysis. Inspection results, quality notifications, and audit findings provide a rich source of information that can inform decision-making at both operational and strategic levels. Candidates must understand how to interpret this data to support continuous improvement and process optimization.
Data-driven decision-making allows organizations to identify recurring defects, monitor supplier performance, and assess the effectiveness of quality interventions. This analytical approach ensures that resources are allocated efficiently, risks are mitigated proactively, and quality objectives are consistently met.
Enhancing Organizational Compliance
Compliance is a critical consideration in modern business environments, and SAP QM supports adherence to regulatory, contractual, and internal standards. By maintaining comprehensive records of inspections, notifications, audits, and certificates, organizations can demonstrate accountability and transparency. Candidates must understand how to configure SAP QM to ensure that compliance requirements are consistently met.
This capability is particularly important in regulated industries such as pharmaceuticals, chemicals, and automotive manufacturing. Compliance not only safeguards the organization from legal and financial penalties but also enhances reputation, customer trust, and market competitiveness.
Continuous Improvement and Feedback Loops
SAP QM is designed to facilitate continuous improvement through structured feedback loops. Inspection results, notifications, and audit findings feed into process optimization initiatives, enabling organizations to refine workflows, reduce defects, and enhance efficiency. Candidates must understand how to leverage these feedback loops to support sustainable quality management practices.
By integrating quality insights with production, procurement, and distribution processes, organizations can achieve proactive quality control. Deviations are addressed promptly, corrective measures are implemented effectively, and operational excellence becomes an ongoing objective rather than a reactive response.
Professional Impact of SAP QM Certification
The C_TPLM40_65 certification equips professionals with the skills needed to implement and manage SAP QM processes effectively. Certified individuals demonstrate proficiency in inspection planning, quality notifications, audits, and integration with other modules. This expertise enables them to contribute meaningfully to organizational quality initiatives and support continuous improvement objectives.
For organizations, employing certified professionals ensures that quality management processes are applied accurately and consistently. Teams gain the ability to monitor compliance, reduce defects, and enhance operational performance, creating tangible benefits in efficiency, reliability, and customer satisfaction.
Strategic Importance of SAP QM
SAP QM is strategically important because it embeds quality considerations across all organizational functions. From procurement to production to delivery, quality management processes ensure that products and services meet customer expectations and regulatory standards. Candidates who understand this strategic dimension are better positioned to influence operational decisions and drive organizational excellence.
By integrating quality management with broader enterprise objectives, organizations can achieve a culture of continuous improvement. Processes are optimized, deviations are minimized, and performance metrics are consistently enhanced. Certification holders play a critical role in fostering this culture, ensuring that quality remains a central organizational priority.
Mastering SAP Quality Management for Discrete Manufacturing
SAP Quality Management plays a critical role in discrete manufacturing, where products are produced in distinct units and quality control must be meticulously maintained at multiple stages. Discrete manufacturing environments often involve complex assembly lines, diverse components, and precise specifications that demand rigorous monitoring. Candidates pursuing the C_TPLM40_65 certification must understand how to configure inspection plans, define quality characteristics, and execute control procedures tailored to these production scenarios.
Inspection lots are central to quality control in discrete manufacturing. These lots allow professionals to systematically evaluate materials, components, or finished products against predefined criteria. By tracking inspection results, assigning responsibility for corrective actions, and integrating findings into broader production workflows, organizations can maintain consistency, reduce defects, and prevent quality deviations from escalating into systemic issues.
SAP QM also enables seamless communication across production teams, ensuring that inspection outcomes are immediately accessible for decision-making. Deviations detected at one stage can trigger notifications or adjustments downstream, creating a feedback loop that reinforces quality throughout the manufacturing process. Mastery of these functionalities is essential for professionals aiming to implement efficient and reliable quality management systems.
Quality Planning in Process Industries
Process industries, including chemicals, pharmaceuticals, and food production, present distinct challenges due to continuous production flows, batch variability, and stringent regulatory requirements. SAP QM provides robust tools for quality planning in these industries, allowing organizations to define inspection methods, sampling procedures, and control strategies aligned with continuous processes.
Candidates preparing for the C_TPLM40_65 exam must understand how to create inspection plans that accommodate the unique characteristics of process industries. Batch management and traceability are essential for maintaining product integrity, enabling organizations to identify, isolate, and address quality deviations effectively. By linking inspection plans with production batches, SAP QM ensures that quality compliance is maintained throughout the manufacturing lifecycle.
Integration with laboratory management and analytics tools further enhances the ability to monitor critical quality attributes. Real-time data collection and analysis allow organizations to detect deviations quickly, implement corrective actions, and optimize processes. Professionals equipped with these skills can contribute significantly to operational efficiency, regulatory compliance, and continuous improvement initiatives.
Leveraging Integration for Operational Excellence
The strategic integration of SAP QM with other modules, including Materials Management, Production Planning, and Sales & Distribution, enables organizations to maintain a seamless quality ecosystem. In procurement, inspection lots generated upon goods receipt ensure that only compliant materials enter production. In manufacturing, quality checks aligned with production schedules allow real-time monitoring of process compliance. For distribution and customer-facing operations, inspections verify that products meet contractual and regulatory requirements before shipment.
Candidates must understand how to configure these integrations, ensuring that quality processes are embedded throughout operational workflows. Automated triggers, notifications, and reporting mechanisms enhance visibility and responsiveness, allowing organizations to address deviations proactively rather than reactively. This interconnected approach fosters operational excellence, reduces defects, and strengthens supply chain reliability.
Automation and Data-Driven Quality Management
Automation is a cornerstone of SAP Quality Management, enabling organizations to streamline workflows, minimize manual errors, and maintain consistent quality standards. Automated inspection lot creation, quality notifications, and certificate generation reduce administrative burden and accelerate decision-making. Candidates preparing for the C_TPLM40_65 certification must understand how to configure these automated processes to achieve maximum efficiency.
Data-driven insights further enhance the effectiveness of SAP QM. Inspection results, audit findings, and notification histories provide valuable information for process optimization. By analyzing patterns, identifying recurring defects, and monitoring supplier performance, organizations can implement targeted improvements and support continuous quality enhancement. Mastery of data interpretation and process analysis ensures that professionals can make informed, strategic decisions that elevate overall quality standards.
Managing Quality Notifications and Corrective Actions
Quality notifications are an essential mechanism for documenting and addressing deviations within SAP QM. When a defect is identified, a notification is generated, capturing relevant information, assigning responsibility, and initiating corrective actions. Candidates must understand the full lifecycle of quality notifications, including classification, prioritization, resolution, and closure.
Effective management of notifications ensures that deviations are addressed promptly and systematically. Integration with other modules allows organizations to link notifications with procurement, production, or distribution activities, creating a coordinated approach to problem-solving. Professionals adept at handling quality notifications can maintain operational continuity, reduce defects, and support continuous improvement objectives.
Corrective actions associated with notifications are critical for sustaining long-term quality standards. SAP QM provides tools to document actions taken, monitor implementation, and evaluate outcomes. By closing the loop between detection and resolution, organizations can reinforce accountability, prevent recurrence, and foster a culture of quality excellence.
Audit Management and Regulatory Compliance
Audit management within SAP QM provides a structured approach to evaluating quality processes and ensuring compliance with internal and external standards. Candidates must understand how to plan, schedule, and execute audits, as well as how to document findings, recommend corrective measures, and track progress. Audits serve as both preventive and corrective tools, identifying weaknesses before they impact operations and ensuring that improvements are sustained over time.
Regulatory compliance is an integral aspect of quality management, particularly in highly regulated industries such as pharmaceuticals, chemicals, and automotive manufacturing. SAP QM enables organizations to maintain comprehensive records of inspections, notifications, and audits, providing traceability and accountability. Professionals who master audit management can help organizations meet stringent regulatory requirements while enhancing operational transparency and credibility.
Source Inspection and Supplier Quality Assurance
Source inspections are a proactive quality measure within SAP QM, allowing organizations to assess supplier quality at the point of origin. By evaluating materials before they enter production, organizations can prevent defective inputs from affecting downstream processes. Candidates must understand how to configure and execute source inspections, including criteria for evaluation, documentation, and follow-up actions.
Supplier quality assurance is closely linked to source inspections, as it involves monitoring, evaluating, and improving supplier performance. SAP QM provides tools to track supplier-related defects, generate notifications, and implement corrective actions. Professionals skilled in supplier quality management can enhance supply chain reliability, reduce defects, and ensure that materials meet organizational and regulatory standards.
Certificates and Documentation for Quality Assurance
Quality certificates in SAP QM provide formal documentation that materials or products meet predefined standards. Candidates must understand how to generate, manage, and interpret certificates, ensuring that they are accurately linked to inspection lots and relevant processes. Certificates serve both internal and external purposes, supporting compliance, traceability, and customer confidence.
Proper management of certificates enhances operational transparency and accountability. It allows organizations to demonstrate adherence to quality standards, streamline reporting processes, and support regulatory audits. Candidates proficient in handling certificates can contribute significantly to the organization’s quality assurance framework and overall operational integrity.
Continuous Improvement and Feedback Loops
SAP QM supports continuous improvement by creating structured feedback loops. Inspection results, notifications, audits, and certificate data feed into process optimization initiatives, allowing organizations to refine workflows, reduce defects, and enhance efficiency. Candidates must understand how to leverage these feedback loops to drive sustainable quality improvements.
By integrating feedback into procurement, production, and distribution processes, organizations can achieve proactive quality control. Deviations are addressed promptly, corrective actions are implemented effectively, and operational excellence becomes a continuous objective. Professionals who understand continuous improvement principles are equipped to foster a culture of quality throughout the enterprise.
Strategic Advantages of SAP QM Certification
The C_TPLM40_65 certification equips professionals with the knowledge and skills required to implement and manage SAP QM processes effectively. Certified individuals demonstrate expertise in inspection planning, quality control, notifications, audits, and integration with other SAP modules. This expertise allows them to contribute meaningfully to organizational quality initiatives, enhance operational efficiency, and support regulatory compliance.
Organizations benefit from employing certified professionals by ensuring that quality management processes are applied accurately and consistently. Certification validates the proficiency of personnel, enhancing credibility, improving decision-making, and strengthening overall operational performance. Teams gain the ability to maintain high-quality standards, reduce defects, and optimize workflows across all functional areas.
Leveraging SAP QM for Competitive Advantage
In competitive industries, SAP Quality Management provides organizations with a strategic advantage by embedding quality processes into every operational layer. From supplier evaluation to production control to customer delivery, SAP QM ensures that products and services consistently meet expectations. Candidates who understand this strategic dimension can influence operational decisions, drive quality initiatives, and contribute to long-term business success.
Automation, integration, and data-driven decision-making enhance the value of SAP QM. By leveraging these capabilities, organizations can reduce errors, optimize processes, and maintain compliance with regulatory and contractual requirements. Certification holders play a vital role in implementing these strategies, ensuring that quality management remains a central organizational priority.
Conclusion
The C_TPLM40_65 certification represents a foundational yet transformative milestone for professionals in SAP Quality Management. It validates a candidate’s ability to navigate and implement core QM processes, including inspection planning, quality control, notifications, audit management, and integration with other SAP modules. Beyond theoretical understanding, the certification emphasizes practical application, preparing candidates to address real-world operational challenges with precision and efficiency.
SAP QM’s comprehensive framework enables organizations to maintain rigorous quality standards across procurement, production, and distribution. Automated inspection lots, source inspections, certificates, and data-driven feedback loops ensure proactive quality oversight, minimize defects, and enhance compliance with regulatory and customer requirements. Certified professionals bring strategic value, fostering continuous improvement, operational efficiency, and accountability.
Ultimately, achieving the C_TPLM40_65 certification equips individuals with the knowledge and skills to drive organizational excellence, support seamless integration of quality processes, and maintain consistent product and service standards, reinforcing both personal growth and enterprise success.
Frequently Asked Questions
Where can I download my products after I have completed the purchase?
Your products are available immediately after you have made the payment. You can download them from your Member's Area. Right after your purchase has been confirmed, the website will transfer you to Member's Area. All you will have to do is login and download the products you have purchased to your computer.
How long will my product be valid?
All Testking products are valid for 90 days from the date of purchase. These 90 days also cover updates that may come in during this time. This includes new questions, updates and changes by our editing team and more. These updates will be automatically downloaded to computer to make sure that you get the most updated version of your exam preparation materials.
How can I renew my products after the expiry date? Or do I need to purchase it again?
When your product expires after the 90 days, you don't need to purchase it again. Instead, you should head to your Member's Area, where there is an option of renewing your products with a 30% discount.
Please keep in mind that you need to renew your product to continue using it after the expiry date.
How often do you update the questions?
Testking strives to provide you with the latest questions in every exam pool. Therefore, updates in our exams/questions will depend on the changes provided by original vendors. We update our products as soon as we know of the change introduced, and have it confirmed by our team of experts.
How many computers I can download Testking software on?
You can download your Testking products on the maximum number of 2 (two) computers/devices. To use the software on more than 2 machines, you need to purchase an additional subscription which can be easily done on the website. Please email support@testking.com if you need to use more than 5 (five) computers.
What operating systems are supported by your Testing Engine software?
Our testing engine is supported by all modern Windows editions, Android and iPhone/iPad versions. Mac and IOS versions of the software are now being developed. Please stay tuned for updates if you're interested in Mac and IOS versions of Testking software.


