Certification: Corrosion and Materials
Certification Full Name: Corrosion and Materials
Certification Provider: API
Exam Code: API-571
Exam Name: Corrosion and Materials
Product Screenshots
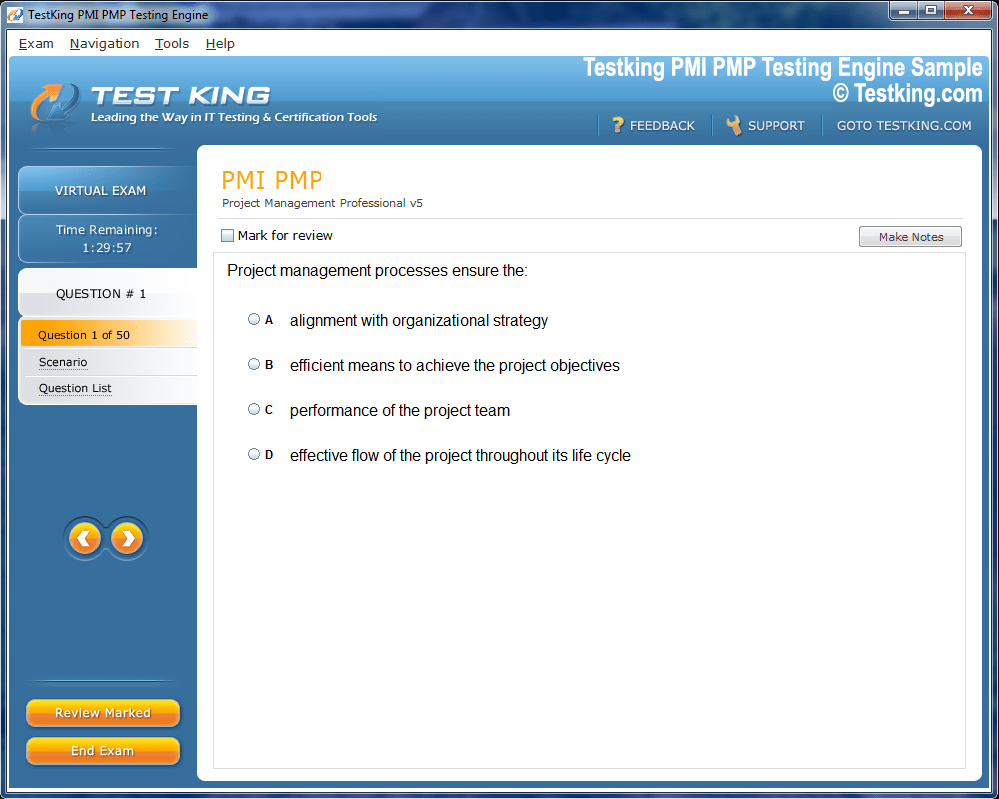
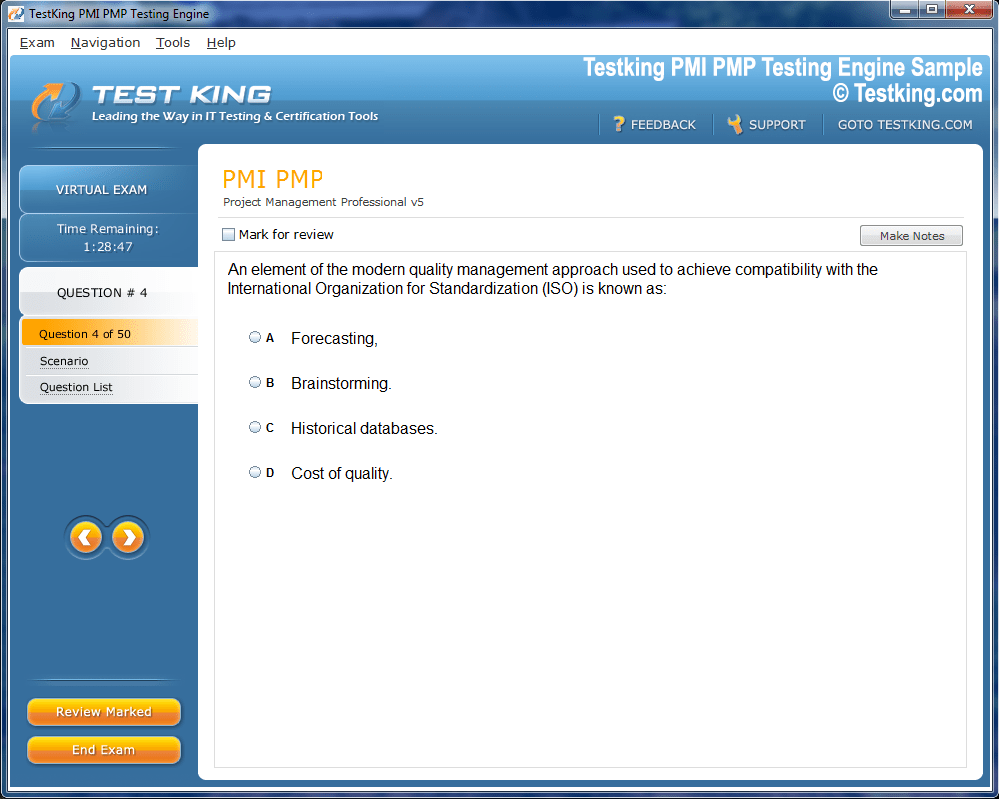
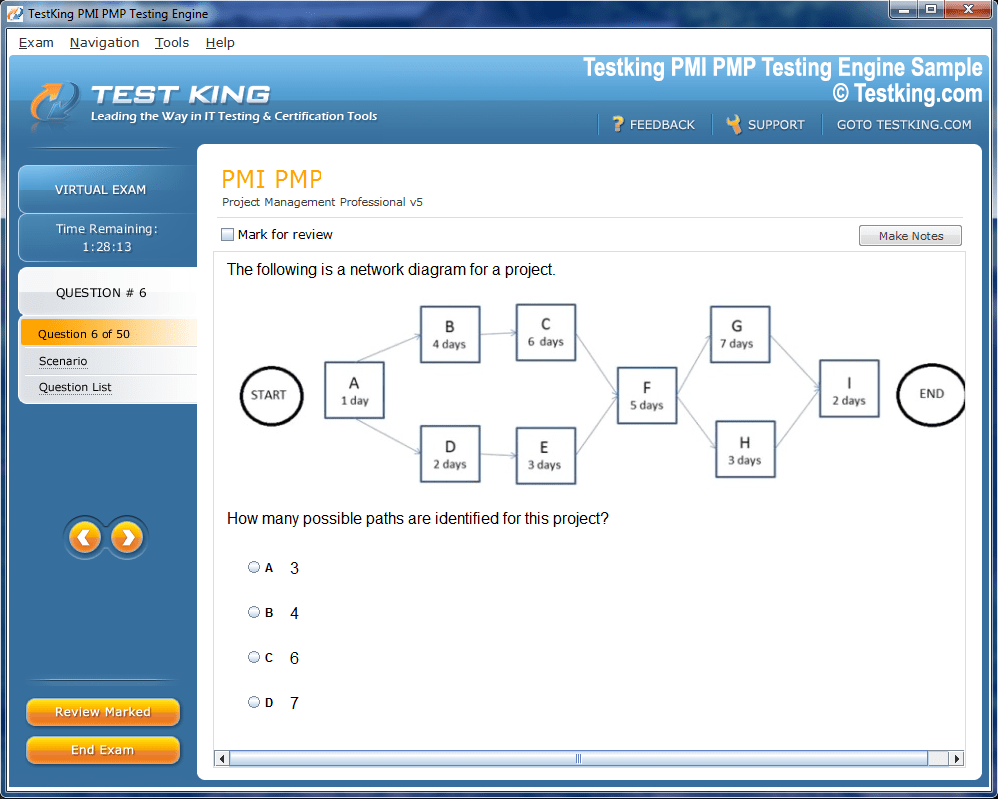
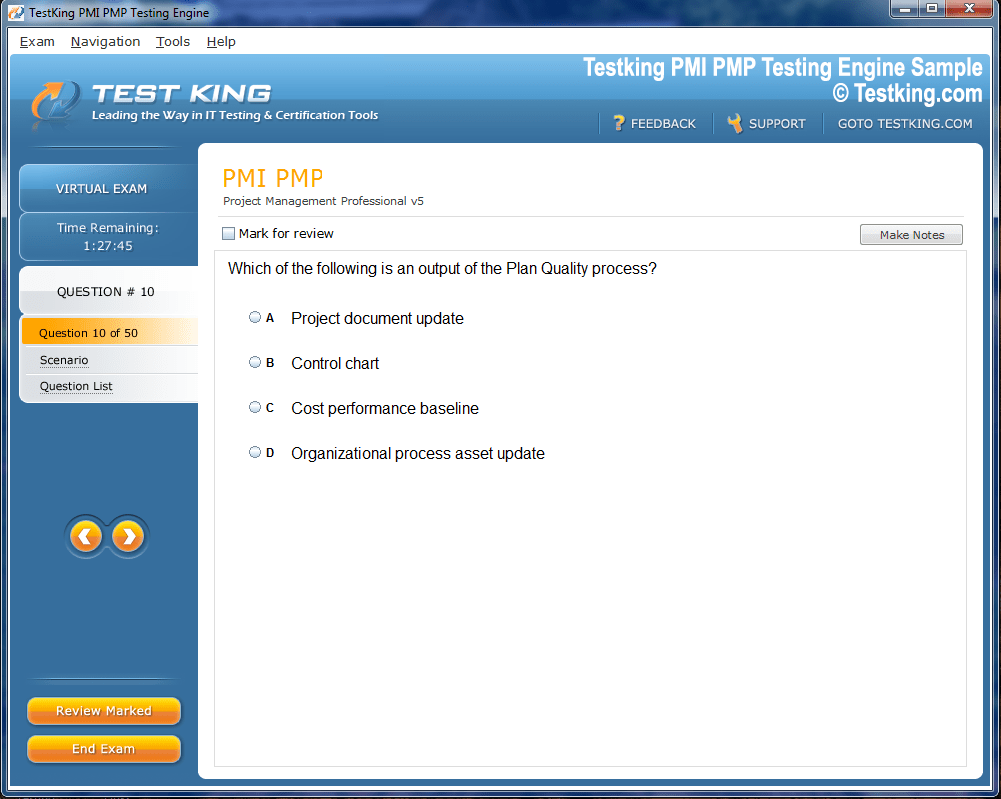
nop-1e =1
Enhancing Asset Life Through Effective Corrosion and Materials Management
Material degradation represents one of the most pervasive challenges confronting contemporary industrial operations worldwide. When discussing asset longevity, operational efficiency, and workplace safety, the intricate relationship between corrosion and materials emerges as a fundamental consideration that cannot be overlooked. This comprehensive exploration delves into the multifaceted nature of material deterioration, examining how various substances respond to environmental stressors and what measures can be implemented to safeguard critical infrastructure.
Industrial sectors ranging from petroleum extraction to maritime transportation face constant battles against the relentless forces of material breakdown. The financial implications alone are staggering, with billions of dollars lost annually due to premature equipment failure, unscheduled maintenance interventions, and catastrophic structural compromises. Beyond economic considerations, the safety ramifications of unchecked material degradation pose serious threats to personnel, communities, and ecosystems.
Understanding the intricate mechanisms that drive material deterioration requires a holistic approach encompassing metallurgy, chemistry, environmental science, and engineering principles. This knowledge forms the foundation upon which effective protection strategies are built, enabling organizations to extend asset lifecycles, optimize maintenance schedules, and maintain operational continuity.
Defining Material Degradation in Industrial Contexts
The phenomenon of material breakdown encompasses a broad spectrum of chemical and electrochemical processes that progressively compromise the structural integrity of substances exposed to reactive environments. This deterioration manifests through various mechanisms, each influenced by distinct environmental parameters, material compositions, and operational conditions.
At its core, material degradation represents the natural tendency of refined substances to revert toward more thermodynamically stable states. Metals extracted from ores and processed into useful forms possess inherent energy that makes them susceptible to reactions with surrounding elements. When these materials encounter moisture, oxygen, acids, or other reactive agents, chemical transformations occur that gradually diminish their mechanical properties and functional capabilities.
The industrial significance of this phenomenon cannot be overstated. Infrastructure components subjected to harsh operating conditions experience accelerated deterioration that threatens their load-bearing capacity, containment functions, and operational reliability. Pipelines transporting corrosive fluids, offshore platforms battered by seawater, chemical processing vessels handling aggressive compounds, and transportation equipment exposed to varied climatic conditions all face ongoing challenges related to material stability.
Recognition of material vulnerability represents the first step toward implementing effective protection measures. Engineers and maintenance professionals must develop comprehensive understanding of how different substances respond to environmental stressors, what warning signs indicate progressive deterioration, and which intervention strategies offer optimal protection for specific applications.
Categories of Susceptible Materials
Material susceptibility to environmental degradation varies significantly based on chemical composition, microstructure, and physical properties. Recognizing these distinctions enables informed material selection and targeted protection strategies. Environmental factors such as moisture, temperature fluctuations, ultraviolet radiation, chemical exposure, and mechanical stresses interact differently with various materials, influencing the rate and type of degradation they may experience.
Metals are particularly vulnerable to corrosion, which is influenced by their electrochemical properties and surface characteristics. For instance, iron and steel are prone to rust formation in the presence of water and oxygen, while aluminum forms a protective oxide layer that slows further degradation. The presence of impurities, grain boundaries, and alloying elements can either accelerate or mitigate corrosion processes. Environmental conditions, such as marine atmospheres with high salinity or industrial environments with acidic pollutants, further exacerbate metallic deterioration.
Polymers and plastics exhibit susceptibility primarily through photo-degradation, thermal aging, and chemical attack. Ultraviolet radiation can break polymer chains, leading to brittleness and color changes, while elevated temperatures accelerate oxidation and chain scission. Certain solvents, acids, or bases can also attack polymer surfaces, resulting in swelling, cracking, or loss of mechanical integrity. The type of polymer, degree of cross-linking, and presence of stabilizers are critical factors influencing durability.
Ceramics and glass are generally resistant to chemical attack but can be vulnerable to mechanical stress and thermal shock. Porosity, microcracks, and surface flaws can act as initiation sites for fracture under cyclic loading or sudden temperature changes. In aggressive chemical environments, some ceramics may slowly dissolve or undergo surface degradation, impacting both structural integrity and aesthetic quality.
Composites combine different material classes, which can lead to complex degradation mechanisms. Fiber-reinforced composites may suffer from matrix cracking, fiber corrosion, or interfacial debonding when exposed to moisture, UV light, or chemicals. Understanding the interactions between constituent materials is essential to predict long-term performance.
Metallic Substances and Their Vulnerabilities
Metallic materials constitute the backbone of industrial infrastructure, prized for their strength, ductility, and thermal conductivity. However, these same properties make them particularly vulnerable to electrochemical reactions with their surroundings.
Ferrous alloys, including carbon steels and cast irons, represent the most widely utilized metallic materials in industrial applications due to their favorable strength-to-cost ratios. Unfortunately, these materials exhibit pronounced susceptibility to oxidation when exposed to moisture and oxygen. The resulting iron oxide formation, commonly recognized as rust, progressively consumes the base metal, creating porous surface layers that offer diminishing protection and accelerated deterioration rates.
The oxidation process occurs through electrochemical reactions where iron atoms lose electrons and combine with oxygen molecules in the presence of water. This transformation creates hydrated iron oxides that occupy greater volume than the original metal, generating expansive stresses that cause surface scaling and spalling. As protective oxide layers flake away, fresh metal surfaces become exposed to continued attack, perpetuating a self-sustaining cycle of degradation.
Aluminum and its alloys present different behavioral characteristics. While aluminum rapidly forms oxide layers upon atmospheric exposure, these films demonstrate remarkable adhesion and impermeability, effectively passivating the underlying metal against further reaction. This natural protection mechanism enables aluminum's widespread use in applications ranging from aircraft structures to architectural elements. However, certain environmental conditions, particularly exposure to chloride ions or highly alkaline solutions, can compromise these protective films, leading to localized attack patterns.
Copper and its derivatives, including brass and bronze, develop distinctive green patina layers composed of copper carbonates and sulfates. While aesthetically notable, these surface films provide moderate protection against atmospheric exposure. In marine environments or industrial atmospheres containing sulfur compounds, copper alloys may experience accelerated tarnishing and localized penetration.
Stainless steels represent engineered solutions designed to resist degradation through deliberate alloying additions. Chromium content exceeding eleven percent enables formation of stable, self-healing oxide films that provide excellent resistance to atmospheric and aqueous environments. Molybdenum additions further enhance resistance to chloride-induced breakdown, while nickel improves general chemical resistance. Despite these enhanced properties, stainless steels remain susceptible to specific attack modes under certain environmental conditions, particularly when protective oxide films become compromised.
Polymeric Materials and Environmental Stress Responses
Polymeric substances, encompassing plastics, elastomers, and synthetic composites, serve increasingly critical roles in modern industrial applications. These materials offer advantages including chemical resistance, lightweight construction, and design flexibility. However, they exhibit distinct vulnerability patterns compared to metallic counterparts.
Environmental stress cracking represents a primary concern for polymeric materials, particularly thermoplastics subjected to mechanical loading in the presence of aggressive chemical agents. This phenomenon manifests as crazing or crack formation under stress levels well below the material's normal failure threshold. The combined effects of tensile stress and chemical exposure create localized molecular chain scission and void formation, propagating through the material structure and eventually leading to catastrophic failure.
Ultraviolet radiation poses another significant threat to polymeric integrity. Photochemical reactions induced by solar exposure cause chain scission and crosslinking modifications that alter mechanical properties. Plasticizer migration, particularly in flexible vinyl compounds, leads to progressive embrittlement as these compounds volatilize or leach into surrounding media. Temperature extremes accelerate these degradation mechanisms, with elevated temperatures promoting oxidative breakdown while subfreezing conditions induce brittle fracture susceptibility.
Certain polymers demonstrate remarkable resistance to specific chemical environments while remaining vulnerable to others. Fluoropolymers exhibit exceptional inertness across broad chemical spectra, enabling their use in highly aggressive service conditions. Conversely, common polyolefins may experience swelling, dissolution, or mechanical property loss when exposed to organic solvents or aromatic hydrocarbons.
Ceramic Materials and Surface Deterioration
Ceramic substances, characterized by their ionic or covalent bonding structures, generally exhibit superior chemical stability compared to metals or polymers. Refractories lining high-temperature vessels, ceramic coatings protecting underlying substrates, and specialized components in corrosive environments all capitalize on these materials' inherent resistance to chemical attack.
Despite their general stability, ceramics remain susceptible to specific degradation modes. Surface erosion through mechanical abrasion gradually wears away protective layers, exposing fresh surfaces to continued attack. Chemical dissolution in strongly acidic or alkaline media can progressively consume certain ceramic compositions. Thermal shock induced by rapid temperature fluctuations generates stress concentrations that propagate microcracks through the material structure, compromising mechanical integrity and creating pathways for environmental ingress.
The brittle nature of ceramic materials amplifies their vulnerability to crack propagation. Unlike ductile metals that accommodate stress through plastic deformation, ceramics concentrate stresses at flaw tips, enabling rapid crack growth under relatively modest loading conditions. This characteristic necessitates careful attention to surface preparation, quality control, and operating condition management to prevent premature failures.
Composite Materials and Interfacial Vulnerabilities
Composite structures combine multiple constituent materials to achieve property combinations unattainable in single-phase substances. Fiber-reinforced polymers, metal matrix composites, and ceramic matrix composites each offer unique performance characteristics suited to specific applications. However, these multi-phase systems introduce additional vulnerability considerations related to interfacial regions and differential material responses.
Galvanic interactions represent a primary concern in composites incorporating dissimilar metals or conductive reinforcements within metallic matrices. When electrically connected materials possessing different electrochemical potentials encounter electrolytic environments, accelerated deterioration of the more active material occurs. Carbon fiber reinforcements within aluminum matrices exemplify this challenge, requiring careful isolation strategies to prevent preferential matrix dissolution.
Interfacial debonding between reinforcement phases and matrix materials creates pathways for environmental ingress into composite structures. Moisture penetration along compromised interfaces degrades adhesive bonds, reduces mechanical property translation from reinforcement to matrix, and creates internal stress concentrations. Freeze-thaw cycling in saturated composites exacerbates this deterioration through repeated expansion-contraction sequences.
The matrix materials in fiber-reinforced composites remain vulnerable to the environmental stress mechanisms affecting bulk polymers. Simultaneously, exposed reinforcement fibers may experience independent degradation through oxidation, dissolution, or stress-assisted cracking. This dual vulnerability necessitates comprehensive protection approaches addressing both constituent materials and their interfacial regions.
Environmental Parameters Influencing Degradation Rates
Material deterioration proceeds at rates governed by complex interactions between environmental conditions, material properties, and mechanical stresses. Recognition of these influencing parameters enables prediction of service life expectations and identification of high-risk operating scenarios.
Temperature Effects on Reaction Kinetics
Thermal energy profoundly influences the rates of chemical and electrochemical reactions driving material degradation. The Arrhenius relationship quantifies this temperature dependency, demonstrating exponential increases in reaction rates with rising temperatures. For many degradation mechanisms, reaction rates approximately double for every ten-degree Celsius temperature increase within moderate temperature ranges.
Elevated operating temperatures accelerate multiple degradation pathways simultaneously. Diffusion rates of reactive species through protective films increase exponentially with temperature, enabling more rapid access to underlying base materials. Oxidation reactions proceed more vigorously, consuming protective oxide layers faster than they regenerate. Mechanical properties deteriorate as materials soften, reducing their resistance to stress-assisted attack mechanisms.
Thermal cycling introduces additional complexity through differential expansion between dissimilar materials, protective coatings, and substrate structures. Repeated heating and cooling generates mechanical stresses at interfaces and within materials exhibiting anisotropic thermal expansion characteristics. These stresses may crack protective barriers, enabling environmental access to vulnerable substrates. Thermal fatigue accumulation progressively weakens materials through microstructural damage accumulation.
Cryogenic conditions present distinct challenges related to material embrittlement and stress concentration. Many structural alloys exhibit ductile-to-brittle transition temperatures below which their fracture toughness decreases dramatically. Operating in these temperature regimes increases susceptibility to catastrophic fracture under impact or shock loading. Condensation and ice formation create additional concerns through moisture concentration and mechanical damage from ice expansion.
Moisture and Humidity Considerations
Aqueous environments enable the electrochemical reactions fundamental to metallic material degradation. Water molecules serve as reaction media, ionic conductors, and reactant participants in the complex series of anodic and cathodic processes constituting electrochemical attack. Even atmospheric moisture at moderate humidity levels provides sufficient aqueous films on material surfaces to sustain active degradation.
Relative humidity thresholds exist below which atmospheric degradation rates decrease substantially. For ferrous materials, critical humidity values typically range between sixty and seventy percent, though this threshold varies with temperature, surface contamination, and atmospheric pollutant concentrations. Above these thresholds, visible moisture films form on material surfaces, establishing continuous electrolytic pathways that accelerate electrochemical reactions.
Condensation phenomena concentrate moisture at vulnerable locations, creating localized environments far more aggressive than bulk atmospheric conditions would suggest. Temperature fluctuations causing condensation deposit contaminated moisture films enriched in chlorides, sulfates, and other aggressive species. These concentrated electrolytes dramatically accelerate attack rates compared to exposures in neutral aqueous solutions.
Immersion conditions represent the most severe moisture exposures, with materials completely surrounded by aqueous media. Dissolved oxygen concentrations, solution chemistry, flow velocities, and temperature combine to determine degradation characteristics. Stagnant conditions beneath deposits or within crevices create occluded environments where localized chemistry evolves independently from bulk solution, often becoming highly aggressive through oxygen depletion and acidification processes.
Chemical Environment Influences
The chemical composition of environments contacting material surfaces exerts profound influence on degradation behavior. Acidic conditions promote rapid metal dissolution through hydrogen evolution reactions that consume base material while generating hydrogen gas. Strong acids overwhelm natural passivation mechanisms, maintaining active dissolution even on alloys typically protected by surface oxide films.
Alkaline environments exhibit selective aggressiveness depending on material composition. Amphoteric metals like aluminum and zinc experience accelerated attack in strongly alkaline solutions as their protective oxides dissolve and material ionization proceeds readily. Conversely, many ferrous alloys demonstrate improved resistance in moderately alkaline conditions where stable oxide films form and persist.
Chloride ions pose particular threats to passive materials, penetrating protective films through localized breakdown mechanisms. Even modest chloride concentrations can initiate pitting attack on stainless steels and aluminum alloys, creating deep, penetrating cavities that propagate autocatalytically once initiated. Marine environments, coastal atmospheres, and process streams containing chloride salts or hydrochloric acid present elevated risks requiring specialized material selections or enhanced protection measures.
Sulfur-containing compounds contribute to degradation through multiple mechanisms. Hydrogen sulfide creates sulfide films on many metals, some of which provide modest protection while others prove porous and non-protective. Sulfuric acid represents one of the most aggressive chemical environments, attacking a broad range of materials through vigorous acid dissolution reactions. Sulfate-reducing bacteria metabolize sulfur compounds while producing hydrogen sulfide and contributing to microbiologically influenced deterioration.
Organic compounds exhibit varied interactions with materials depending on their chemical structures and material susceptibilities. Hydrocarbon streams in petroleum processing operations may contain naphthenic acids that aggressively attack steel alloys at elevated temperatures. Organic solvents swell and dissolve many polymeric materials while presenting minimal concerns for metallic components. Amine solutions used for acid gas removal create specific attack patterns on carbon steels, requiring careful materials engineering and operational control.
Microbiological Contributions to Material Breakdown
Living organisms contribute to material degradation through diverse mechanisms collectively termed microbiologically influenced deterioration. Bacterial colonies, fungal growth, and algae formations create localized environments beneath biofilm deposits where oxygen depletion, acidification, and concentration of aggressive metabolic byproducts accelerate attack on underlying materials.
Sulfate-reducing bacteria thrive in anaerobic environments beneath deposits or within soil contact regions. These organisms metabolize sulfate ions, producing hydrogen sulfide as a metabolic byproduct. The resulting sulfide corrosion mechanisms prove particularly damaging to ferrous materials, with rapid penetration rates exceeding those observed in comparable abiotic environments.
Acid-producing bacteria excrete organic acids including acetic, formic, and citric acids during normal metabolic processes. Accumulation of these compounds beneath biofilm deposits creates localized acidification that attacks protective films and promotes active metal dissolution. In combination with oxygen concentration cells formed by biofilm respiration, these organisms generate aggressive microenvironments far exceeding bulk solution aggressiveness.
Iron-oxidizing bacteria accelerate electrochemical reactions through enzymatic oxidation of ferrous ions to ferric states. This biological catalysis enhances cathodic reactions while establishing favorable conditions for continued anodic dissolution. The resulting deterioration patterns often exhibit distinctive tubercle formations covering active pits where bacterial colonies concentrate their metabolic activities.
Fungal growth on organic coatings and polymeric materials degrades these protective barriers through enzymatic breakdown of polymer chains and mechanical disruption from hyphae penetration. Water retention within fungal colonies maintains localized moisture conditions favoring continued growth and underlying material attack. Organic acid excretion contributes additional aggressiveness, particularly on metallic substrates beneath compromised coating systems.
Strategic Approaches to Degradation Prevention
Effective material protection requires comprehensive strategies incorporating multiple defensive layers. No single approach provides complete protection across all operating conditions and timeframes. Instead, successful programs implement complementary measures addressing immediate threats while establishing monitoring frameworks ensuring long-term integrity.
Surface Barrier Systems
Protective coatings represent the most widely implemented degradation prevention strategy, interposing barrier layers between vulnerable substrates and aggressive environments. Proper coating system design, application, and maintenance deliver cost-effective protection spanning years or decades of service.
Organic coatings encompass diverse formulations tailored to specific exposure conditions and performance requirements. Epoxy systems offer excellent adhesion, chemical resistance, and moisture barrier properties suitable for immersion and chemical processing applications. Polyurethane topcoats provide outstanding weatherability and gloss retention for atmospheric exposures. Zinc-rich primers deliver sacrificial protection to steel substrates through galvanic mechanisms, functioning as applied anodes that preferentially corrode while protecting base metal.
Coating system effectiveness depends critically on surface preparation quality preceding application. Contaminants including mill scale, rust, oils, and soluble salts must be thoroughly removed to establish clean, profiled surfaces promoting mechanical adhesion. Abrasive blast cleaning represents the gold standard surface preparation method, simultaneously removing contaminants and creating anchor patterns enhancing coating adhesion. Surface cleanliness and profile depth specifications ensure consistent preparation quality supporting coating system performance.
Application parameters including environmental conditions, material temperatures, film thickness, and curing schedules significantly influence coating performance and longevity. Humidity, temperature, and dew point considerations prevent moisture entrapment and ensure proper curing reactions. Film thickness measurements verify adequate barrier properties while avoiding excessive buildups causing cohesive weakness or application defects. Multi-coat systems incorporate primers, intermediate coats, and topcoats, each serving specific functions within the overall protective scheme.
Metallic coatings including hot-dip galvanizing, thermal spray applications, and electroplated deposits provide durable protection through barrier and galvanic mechanisms. Zinc coatings on ferrous substrates offer dual protection, initially functioning as barriers while later providing cathodic protection as coatings weather and zinc becomes exposed. Aluminum thermal spray coatings demonstrate excellent performance in high-temperature applications where organic coatings would rapidly degrade. These inorganic systems tolerate elevated operating temperatures while maintaining protective characteristics throughout extended service lives.
Ceramic and refractory coatings protect components operating in extreme temperature, erosive, or chemically aggressive environments exceeding organic coating capabilities. Alumina, chromia, and carbide coatings applied through thermal spray, physical vapor deposition, or chemical vapor deposition techniques create hard, chemically inert surfaces resisting multiple degradation mechanisms simultaneously. These systems find applications in combustion environments, chemical processing vessels, and wear-resistant components where material preservation under extreme conditions justifies higher initial costs.
Coating maintenance and inspection programs prove essential for realizing theoretical service life expectations. Periodic inspections identify coating degradation including blistering, cracking, delamination, and mechanical damage requiring prompt repair. Small damaged areas repaired expeditiously prevent underlying material attack and coating system undermining that progressively expands damaged zones. Maintenance recoating intervals established through monitoring programs ensure protective integrity throughout asset operational lives.
Electrochemical Protection Methodologies
Cathodic protection systems control electrochemical reactions driving metallic degradation through externally applied electrical currents or sacrificial anode installations. These techniques prove particularly valuable for structures in conductive environments including buried pipelines, marine structures, internal surfaces of storage tanks, and reinforcing steel within concrete.
Sacrificial anode systems utilize more active metals including zinc, magnesium, and aluminum alloys connected electrically to protected structures. Galvanic potential differences between anode materials and protected structures drive protective currents suppressing anodic dissolution reactions on structure surfaces. Anode materials corrode preferentially, requiring periodic replacement as they consume. System design parameters including anode quantity, distribution, and output characteristics ensure adequate current distribution across protected surfaces.
Impressed current cathodic protection employs external power sources driving protective currents from strategically positioned anode installations to protected structures. This approach enables protection of larger structures, offers adjustable current outputs accommodating changing protection requirements, and utilizes dimensionally stable anode materials requiring infrequent replacement. Control systems automatically adjust output currents maintaining optimal protection levels while minimizing overprotection risks including hydrogen embrittlement and coating disbondment.
Protection criterion establishment requires careful consideration of material characteristics, environmental conditions, and operational constraints. Potential measurements using reference electrodes positioned on protected surfaces assess protection adequacy, with specified negative potential shifts relative to freely corroding conditions indicating effective protection. Polarization behavior studies inform criterion selection, balancing adequate protection against overprotection consequences.
Cathodic protection system monitoring includes periodic potential surveys verifying adequate protection across all structure regions, inspection of rectifier installations ensuring proper operation, and anode consumption assessments determining replacement timing. Automated monitoring systems incorporating data logging and remote communication capabilities enable continuous performance verification and prompt identification of system abnormalities requiring corrective action.
Anodic protection represents a specialized technique applicable to materials exhibiting active-passive behavior in specific chemical environments. External current application maintains material surfaces within passive potential ranges where stable, protective oxide films persist. This approach proves valuable for protecting steel and stainless steel storage vessels containing corrosive chemicals including sulfuric acid, caustic solutions, and nitric acid. Precise potential control requirements and consequences of system failures necessitate sophisticated controls and redundant safety measures.
Material Selection Engineering
Specifying corrosion-resistant materials appropriately matched to anticipated service conditions represents a fundamental prevention approach avoiding degradation concerns through inherent material resistance. This strategy proves particularly valuable for critical components where coating damage risks are high, maintenance access proves difficult, or operational reliability requirements demand maximum assurance.
Stainless steel selection encompasses numerous grades offering varied resistance levels to different environments. Austenitic grades including Types 304 and 316 provide good general resistance to atmospheric and many aqueous environments. Molybdenum-bearing grades demonstrate enhanced chloride resistance reducing pitting and crevice corrosion susceptibility in marine and brackish water exposures. Ferritic stainless steels offer stress corrosion cracking resistance advantages in chloride-containing environments while limiting nickel content for economic or material property considerations.
Nickel-based alloys provide exceptional resistance to extremely aggressive environments including hot, concentrated acids, high-temperature oxidizing conditions, and sour petroleum production streams. Alloy selection within this family considers specific environmental challenges including oxidizing versus reducing conditions, presence of chlorides, temperature ranges, and required mechanical properties. These premium materials justify their costs in applications where alternatives would experience rapid degradation requiring frequent replacement.
Titanium and its alloys demonstrate outstanding resistance to chloride-containing environments including seawater, hypochlorite solutions, and wet chlorine. The stable, protective oxide films forming on titanium surfaces provide immunity to pitting and crevice corrosion over broad temperature ranges. Applications in marine heat exchangers, desalination plants, and chemical processing equipment capitalize on titanium's unique combination of corrosion resistance, strength, and light weight. Temperature limitations above approximately 300 degrees Celsius restrict applications requiring combined high-temperature and corrosion resistance.
Non-metallic materials including fiber-reinforced polymer composites, fluoropolymers, and advanced ceramics enable operation in environments incompatible with any metallic materials. Chemical processing equipment handling highly acidic or alkaline solutions, high-purity pharmaceutical and semiconductor manufacturing operations, and specialized applications benefit from the broad chemical resistance these materials provide. Design considerations address their distinct mechanical property characteristics including lower strength-to-weight ratios, temperature limitations, and brittle fracture susceptibility.
Material qualification testing verifies suitability for intended services through exposure to simulated or actual operating conditions. Laboratory immersion testing, electrochemical evaluation, and mechanical property assessments after environmental exposure identify potential vulnerabilities before field deployment. Pilot-scale trials in actual operating environments confirm performance predictions while identifying unanticipated factors requiring design modifications. Systematic qualification programs reduce risks of premature failures from inappropriate material selections while building knowledge bases informing future designs.
Design Configuration Optimization
Equipment and structural design configurations profoundly influence degradation susceptibility through their effects on environmental exposure severity, drainage characteristics, and accessibility for inspection and maintenance. Thoughtful design attention during project conception provides lasting benefits throughout asset operational lives.
Crevice minimization represents a fundamental design principle limiting localized attack initiation sites. Butt-welded connections prove superior to bolted flanges for buried or immersed piping systems, eliminating crevice formers where aggressive chemistries develop. Continuous welds rather than intermittent attachments reduce crevice occurrence on fabricated structures. Gasket selections and sealing surface finishes influence crevice geometries and subsequent localized attack susceptibility.
Drainage provision prevents moisture accumulation in vulnerable locations through gravity flow and strategic drain hole placement. Horizontal surfaces with slight inclination eliminate standing water promoting localized attack. Low-point drains remove condensate and entrained liquids from vessels and piping systems. Elimination of U-shaped configurations where solids settle and liquids accumulate reduces localized environmental severity and deposit-related deterioration.
Stress concentration minimization through generous fillet radii, smooth transitions, and elimination of sharp corners reduces susceptibility to stress-assisted degradation modes including stress corrosion cracking and corrosion fatigue. Notch effects amplifying local stress levels create preferential initiation sites for environmentally assisted cracking mechanisms. Careful attention to welding details, machining operations, and assembly procedures prevents introduction of detrimental stress concentration features.
Galvanic isolation between dissimilar metals prevents accelerated deterioration of more active materials through electrical insulation at connections. Insulating gaskets, bushings, and coatings interrupt galvanic circuits while maintaining structural continuity. Area ratio considerations inform material pairing decisions, recognizing that unfavorable area ratios where small anodic surfaces contact large cathodic surfaces create particularly aggressive galvanic scenarios. Material selection emphasis on compatible materials within the galvanic series reduces driving forces for preferential dissolution.
Accessibility features incorporated during design facilitate inspection and maintenance activities throughout operational lives. Removable insulation sections, permanent access platforms, and inspection ports enable detailed condition assessments without requiring extensive scaffold erection or insulation removal. Ultrasonic testing access points on piping systems permit wall thickness monitoring documenting degradation rates and remaining service life. Maintenance-friendly designs acknowledging human factors and practical field constraints realize superior long-term integrity compared to designs optimized solely for initial construction efficiency.
Environmental Control Strategies
Modifying service environments to reduce aggressiveness provides another degradation prevention approach applicable in specific situations. These strategies address root causes of degradation rather than merely protecting materials from hostile conditions, potentially delivering superior long-term results.
Dehumidification systems controlling atmospheric moisture levels below critical thresholds prevent electrochemical reactions requiring aqueous films. Enclosed spaces including storage buildings, electronic equipment enclosures, and preservation packaging utilize desiccants, refrigeration, or mechanical dehumidification maintaining specified relative humidity limits. Humidity monitoring and control systems ensure continuous protection throughout storage or operational periods.
Chemical inhibitor additions to process streams, cooling waters, and aqueous systems modify solution chemistry suppressing anodic dissolution reactions, cathodic oxygen reduction, or both simultaneously. Filming inhibitors including organic amines deposit protective molecular layers on metal surfaces impeding charge transfer processes. Oxidizing inhibitors including chromates and nitrites promote passive film formation maintaining materials within protected potential ranges. Volatile corrosion inhibitors sublime from solid or liquid formulations, condensing on metal surfaces within enclosed spaces to provide vapor-phase protection.
Oxygen removal from aqueous systems eliminates the primary cathodic reactant supporting most degradation mechanisms in neutral and alkaline solutions. Mechanical deaeration through vacuum or steam stripping, chemical scavenging using sulfites or hydrazine, and membrane separation technologies reduce dissolved oxygen to trace levels unable to sustain significant degradation rates. Closed systems with nitrogen or inert gas blanketing prevent oxygen ingress maintaining deaerated conditions throughout operation.
pH control through chemical adjustment maintains aqueous environments within ranges where materials demonstrate optimal resistance. Alkalinity additions to condensate systems prevent carbonic acid attack on steel components. Acid neutralization in process streams protects equipment from aggressive low-pH conditions. Buffering systems maintain stable pH levels despite process variations or contaminant ingress that might otherwise shift chemistry toward more aggressive regimes.
Temperature control limitations restricting maximum operating temperatures below thresholds where degradation rates accelerate dramatically extend equipment service lives. Cooling systems, insulation, and operational procedures maintaining components within specified temperature ranges prevent thermally activated attack modes. Process modifications reformulating chemical compositions or operating conditions may enable less severe thermal exposures without sacrificing production objectives.
Monitoring and Inspection Techniques
Systematic monitoring programs detecting degradation during early stages enable timely interventions preventing failures while optimizing maintenance resource allocation. Diverse inspection technologies assess material condition from various perspectives, each offering distinct advantages for specific applications and degradation modes.
Visual Examination Approaches
Direct visual inspection represents the simplest and most frequently applied assessment method, identifying surface anomalies including coating breakdown, rust formation, deposit accumulation, and mechanical damage. Systematic examination protocols ensure comprehensive coverage of accessible surfaces while documenting findings through written reports and photographic records. Portable borescopes extend visual examination capabilities into internal cavities and restricted access locations including vessel interiors, small-diameter piping, and confined spaces.
Enhanced visual inspection incorporates magnification and specialized lighting revealing subtle surface features invisible during casual observation. Magnifying glasses, portable microscopes, and video microscopy systems enable detailed examination of crack-like indications, surface texture changes, and coating microstructure. Ultraviolet lighting reveals organic residues, coating discontinuities, and biological growth patterns difficult to discern under white light illumination.
Remote visual inspection utilizing robotic vehicles, remotely operated platforms, and unmanned aerial vehicles provides access to hazardous or difficult-to-reach locations without requiring personnel entry or extensive scaffolding. Pipeline inspection technologies traverse internal passages documenting internal surface conditions through video imaging and specialized sensor arrays. Drone-based examinations survey external surfaces of tall structures, elevated piping racks, and extensive roof areas efficiently while minimizing safety risks and operational disruptions.
Non-Destructive Testing Technologies
Ultrasonic thickness measurements quantify remaining wall thickness at specific locations, documenting material loss from general or localized degradation mechanisms. Measurements at established reference points tracked over time establish degradation rate trends informing remaining service life predictions. Thickness mapping surveys across broad areas identify localized thinning patterns requiring enhanced monitoring or repair interventions. Advanced phased array ultrasonic systems generate detailed thickness profiles revealing complex degradation patterns including preferential weld attack and flow-accelerated corrosion.
Magnetic particle inspection detects surface-breaking and shallow subsurface discontinuities in ferromagnetic materials through magnetic field distortions at defect locations. Dry particle, wet fluorescent particle, and permanent magnet techniques suit different field conditions and sensitivity requirements. Applications include weld examination, crack detection in highly stressed components, and verification of repair quality. Limitations to ferromagnetic materials restrict applicability, while surface preparation requirements and careful technique execution prove critical for reliable results.
Liquid penetrant examination reveals surface-breaking defects in non-porous materials through capillary action drawing colored or fluorescent penetrants into discontinuity openings. Subsequent developer applications pull penetrant from defects, creating visible indications for examiner evaluation. Versatility across diverse material types including metals, ceramics, plastics, and composites broadens applicability. Portability and simplicity enable field examinations without requiring extensive equipment or electrical power. Detection limitations to surface-breaking defects represent the primary technique constraint.
Radiographic examination creates shadow images recording internal discontinuities, wall thickness variations, and assembly configurations. X-ray and gamma-ray sources suit different accessibility constraints and material thickness ranges. Digital radiography techniques offer enhanced sensitivity, reduced exposure times, and immediate image availability compared to traditional film radiography. Applications span weld quality verification, casting integrity assessment, and detection of internal erosion or deposit accumulation in piping systems. Radiation safety considerations necessitate careful planning, area access controls, and dosimetry programs protecting personnel from exposure hazards.
Eddy current testing induces electrical currents in conductive materials, detecting surface and near-surface anomalies through electromagnetic interactions. Probe designs tailored to specific applications enable rapid scanning of large areas, detailed examination of fastener holes, and heat exchanger tube inspection from internal surfaces. Sensitivity to material property variations including conductivity and permeability changes aids heat treatment verification and material identification. Surface preparation requirements prove minimal compared to magnetic particle or penetrant techniques. Depth of penetration limitations restrict detection to near-surface regions, while complex signal interpretation requires experienced personnel.
Acoustic emission monitoring detects stress waves generated by active crack growth, fiber breakage in composites, or coating delamination during structural loading. Sensor arrays strategically positioned on structures record emission events, with source location algorithms triangulating defect positions from arrival time differences at multiple sensors. Real-time monitoring during proof testing or operational loading identifies active damage mechanisms requiring further evaluation. Ability to assess large structure volumes from limited sensor positions proves advantageous for pressure vessels, piping systems, and storage tanks. Interpretation complexity and requirement for active defect growth during monitoring represent primary limitations.
Electrochemical Assessment Methods
Potential measurements using reference electrodes contacting material surfaces or positioned within electrolytic environments assess thermodynamic driving forces for degradation reactions. Half-cell potential surveys map potential distributions across structures revealing areas experiencing active degradation or inadequate cathodic protection. Continuous potential monitoring detects sudden potential shifts indicating coating damage, cathodic protection system malfunctions, or environmental changes affecting degradation susceptibility.
Linear polarization resistance measurements quantify instantaneous degradation rates through controlled electrochemical perturbations and response analysis. Portable instrumentation enables field measurements on operating equipment without requiring extended shutdowns or sample removal. Periodic monitoring at established reference locations tracks degradation rate variations over time, documenting seasonal influences, operational condition effects, and inhibitor effectiveness. Small measurement areas provide localized information rather than structure-averaged assessments.
Electrochemical impedance spectroscopy applies alternating current signals across frequency ranges, analyzing impedance responses revealing coating barrier properties, interfacial reactions, and charge transfer resistances. Sophisticated data analysis extracts multiple parameters characterizing system behavior including coating capacitance, pore resistance, and charge transfer resistance. Laboratory studies inform protective mechanism understanding while field measurements assess coating aging and degradation progression. Equipment complexity and measurement duration constraints limit routine field application compared to simpler techniques.
Electrochemical noise monitoring records spontaneous potential and current fluctuations arising from degradation processes, analyzing signal characteristics identifying pitting, uniform dissolution, or passivity. Continuous monitoring installations track degradation mode transitions and intensity variations correlating with operational parameters. Diagnostic capabilities differentiating degradation mechanisms prove valuable for optimizing inhibitor strategies and validating material selection decisions. Interpretation sophistication and sensor maintenance requirements exceed those for simpler electrochemical techniques.
Sampling and Laboratory Analysis
Coupon exposure programs suspend standardized test specimens within operating environments, retrieving them periodically for laboratory examination quantifying degradation rates and mechanisms. Weight loss measurements following cleaning procedures per standardized protocols provide quantitative degradation rate data. Metallographic examination reveals microstructural damage, attack morphology, and depth of penetration. Surface analysis identifies corrosion products informing mechanism diagnosis. Multiple coupon materials enable comparative evaluations identifying optimal specifications for equipment repairs or upgrades.
Deposit analysis characterizes accumulation chemistry, physical properties, and microbiological populations contributing to localized degradation beneath deposits. Chemical analysis determines major and minor constituents including chlorides, sulfates, organic acids, and metal sulfides. Microbiological enumeration quantifies bacterial populations including sulfate-reducing bacteria, acid-producing bacteria, and general heterotrophic organisms. Physical characterization assesses deposit adhesion, porosity, and moisture retention properties. Findings guide operational modifications reducing deposition tendencies or chemical cleaning programs removing existing accumulations.
Water and process fluid sampling enables laboratory analysis tracking chemistry variations affecting degradation characteristics. Ion chromatography quantifies dissolved chlorides, sulfates, and organic acids. pH and conductivity measurements document acidity and ionic strength. Dissolved gas analysis including oxygen, carbon dioxide, and hydrogen sulfide assesses cathodic reactants and acidifying species. Microbiological testing identifies contamination requiring biocide treatment. Trending analysis detects gradual composition shifts or sudden upsets requiring corrective action preventing equipment damage.
Conclusion
The enhancement of asset life through effective corrosion and materials management is not merely a technical necessity but a strategic imperative for organizations seeking to optimize operational efficiency, reduce costs, and ensure long-term sustainability. Corrosion, a pervasive and often underestimated challenge, poses significant risks to structural integrity, safety, and performance across industries. Left unaddressed, it can lead to unexpected failures, costly downtime, and substantial financial losses. Therefore, a proactive and systematic approach to corrosion management, underpinned by sound materials selection, maintenance practices, and monitoring technologies, is essential for safeguarding assets and maximizing their service life.
A key aspect of effective corrosion management is the integration of materials science into asset planning and design. Selecting materials that exhibit high resistance to the specific environmental conditions in which they operate—such as high humidity, saline exposure, or chemical aggressiveness—forms the foundation for long-term durability. Coupled with protective measures such as coatings, cathodic protection, and environmental controls, these material choices significantly reduce the rate of degradation and minimize the likelihood of unexpected failures. This approach highlights the importance of understanding both the operational environment and the inherent properties of the materials in use, enabling engineers to make informed decisions that balance performance, cost, and longevity.
Moreover, the implementation of predictive maintenance and condition monitoring technologies plays a critical role in extending asset life. Techniques such as non-destructive testing, corrosion mapping, and real-time sensor monitoring allow for early detection of deterioration, enabling timely interventions before significant damage occurs. By shifting from reactive maintenance to a predictive and preventive paradigm, organizations can not only mitigate risks but also optimize resource allocation, reduce operational disruptions, and enhance overall safety. This proactive methodology underscores the value of data-driven decision-making in modern asset management strategies.
Training and organizational culture are equally important in achieving effective corrosion and materials management. Personnel must be equipped with the knowledge and skills to identify potential issues, apply appropriate protective measures, and adhere to best practices consistently. A culture that prioritizes asset integrity, continuous monitoring, and proactive maintenance ensures that management strategies are not only designed but effectively implemented throughout the asset lifecycle.
Finally, the economic and environmental benefits of extending asset life cannot be overstated. Reducing the frequency of replacements, minimizing material waste, and avoiding catastrophic failures contribute to significant cost savings and sustainable operations. Industries that embrace comprehensive corrosion and materials management frameworks are better positioned to achieve operational excellence, comply with regulatory standards, and meet sustainability goals.
Enhancing asset life through effective corrosion and materials management is a multifaceted endeavor that combines material science, engineering practices, predictive technologies, and organizational commitment. It requires foresight, continuous monitoring, and strategic investment in both knowledge and technology. Organizations that adopt a holistic, proactive approach can significantly extend the service life of their assets, improve safety and reliability, reduce operational costs, and achieve long-term sustainability. Ultimately, effective corrosion and materials management is not just a technical strategy—it is a cornerstone of responsible, efficient, and forward-looking asset stewardship.
Frequently Asked Questions
Where can I download my products after I have completed the purchase?
Your products are available immediately after you have made the payment. You can download them from your Member's Area. Right after your purchase has been confirmed, the website will transfer you to Member's Area. All you will have to do is login and download the products you have purchased to your computer.
How long will my product be valid?
All Testking products are valid for 90 days from the date of purchase. These 90 days also cover updates that may come in during this time. This includes new questions, updates and changes by our editing team and more. These updates will be automatically downloaded to computer to make sure that you get the most updated version of your exam preparation materials.
How can I renew my products after the expiry date? Or do I need to purchase it again?
When your product expires after the 90 days, you don't need to purchase it again. Instead, you should head to your Member's Area, where there is an option of renewing your products with a 30% discount.
Please keep in mind that you need to renew your product to continue using it after the expiry date.
How often do you update the questions?
Testking strives to provide you with the latest questions in every exam pool. Therefore, updates in our exams/questions will depend on the changes provided by original vendors. We update our products as soon as we know of the change introduced, and have it confirmed by our team of experts.
How many computers I can download Testking software on?
You can download your Testking products on the maximum number of 2 (two) computers/devices. To use the software on more than 2 machines, you need to purchase an additional subscription which can be easily done on the website. Please email support@testking.com if you need to use more than 5 (five) computers.
What operating systems are supported by your Testing Engine software?
Our testing engine is supported by all modern Windows editions, Android and iPhone/iPad versions. Mac and IOS versions of the software are now being developed. Please stay tuned for updates if you're interested in Mac and IOS versions of Testking software.


